Beta-mannase mutant Man5AS11R with improved heat resistance and specific activity and encoding gene thereof
A mannanase and specific activity technology, applied in the fields of biotechnology and enzyme engineering, can solve the problems of low enzyme activity, unsatisfactory heat resistance and enzyme activity, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
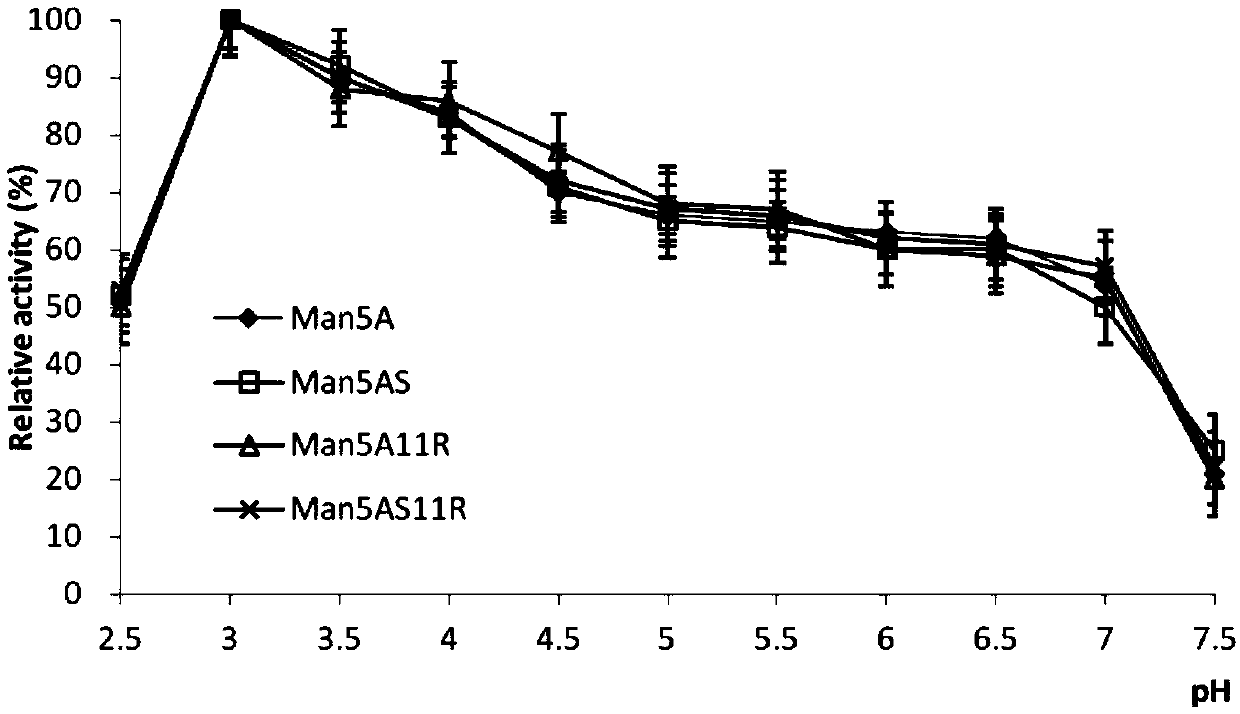
[0017] Example 1 Optimal pH and pH Stability of Thermostable Acidic β-Mannanase Mutants
[0018] The wild-type thermostable acid β-mannanase Man5A was determined and analyzed respectively for its mutants Man5AS (K16C and D296C), Man5A11R (R21V, I80D and I218E) and Man5AS11R (K16C, R21V, I80D, I218E and D296C). suitable for pH, the result is as figure 1 As shown, the optimum pH of each mutant is 3, and the change trend of its relative enzyme activity between pH 2.5-7.5 is consistent with that of Man5A. It can be seen that the mutation site of each mutant does not change the optimal activity of the enzyme. Suitable for pH.
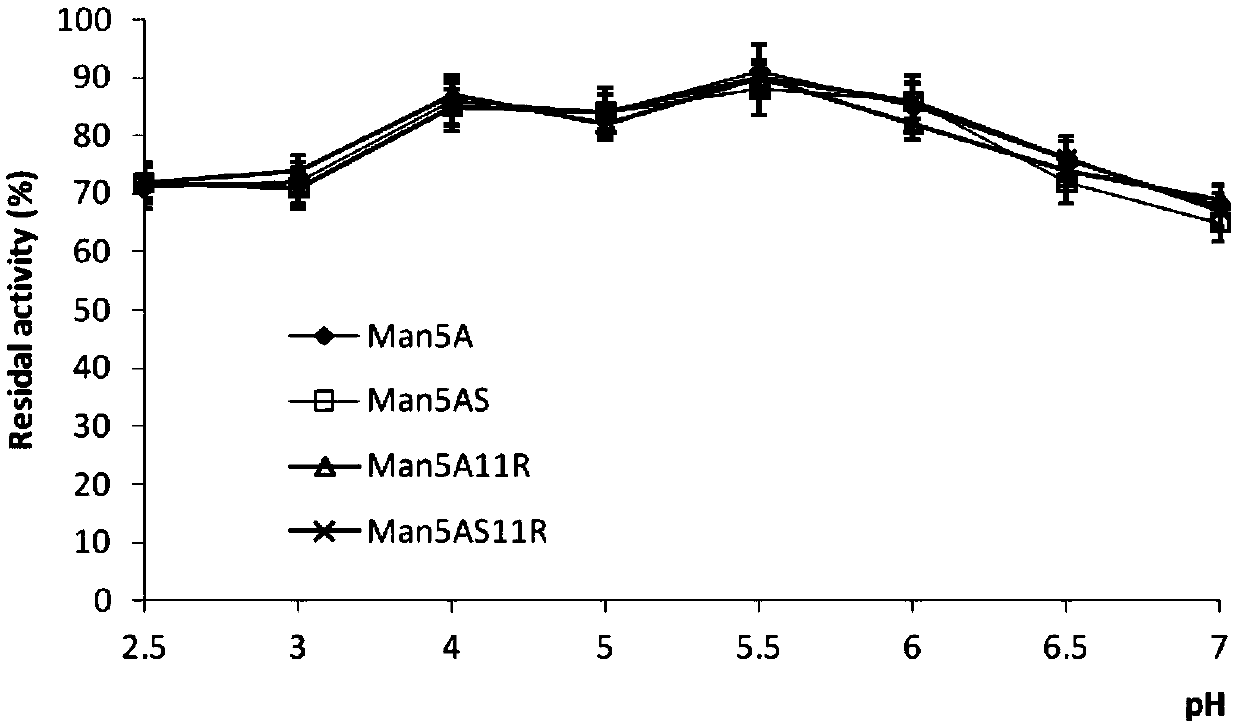
[0019] As a control wild-type heat-resistant acidic β-mannanase Man5A, the mutants Man5AS (K16C and D296C), Man5A11R (R21V, I80D and I218E) and Man5AS11R (K16C, R21V, I80D, I218E and D296C) were analyzed at pH 2 respectively. .5-7 pH stability preserved for 1h, the results are as follows figure 2 As shown, the change trend of the residual enzyme acti...
Embodiment 2
[0020] Example 2 Optimum Action Temperature and Temperature Stability of Thermostable Acidic β-Mannanase Mutants
[0021] Control wild-type heat-resistant acid β-mannanase Man5A, respectively assay and analyze its mutants Man5AS (K16C and D296C), Man5A11R (R21V, I80D and I218E) and Man5AS11R (K16C, R21V, I80D, I218E and D296C) Optimum temperature, the results are as follows image 3 As shown, the optimum action temperature of each mutant is 60 o C, where, mutants Man5AS and Man5AS11R at 80 o The relative enzymatic activity of C was increased, while the mutant Man5A11R was consistent with Man5A. It can be seen that the introduction of intramolecular disulfide bonds can improve the relative enzymatic activity of the enzyme under high temperature conditions.
[0022] As a control wild-type heat-resistant acidic β-mannanase Man5A, the mutants Man5AS (K16C and D296C), Man5A11R (R21V, I80D and I218E) and Man5AS11R (K16C, R21V, I80D, I218E and D296C) were analyzed at 40 -80 o ...
Embodiment 3
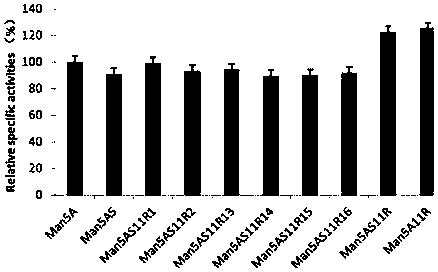
[0023] Example 3 Increased Specific Activity of Thermostable Acidic β-Mannanase Mutants
[0024] On the basis of the mutant enzyme Man5AS, the present invention introduces three point mutations of R21V, I80D and I218E to further improve its specific activity. At the same time, in order to investigate the influence of the three mutation points on the activity of the enzyme, as shown in Table 1, each The specific activity of the mutants changed as Figure 5 As shown, the specific activities of Man5A11R and Man5AS11R in the mutant were increased by 25.6% and 22.5%, respectively, compared with the wild enzyme Man5A, but as image 3 and Figure 4 As shown, the heat resistance of Man5A11R was not significantly improved, while Man5AS11R was improved in both heat resistance and specific activity. At the same time, the present invention also found that on the basis of Man5AS, only one or two mutation sites in Man5A11R were mutated, but the specific activity was not improved. It ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com