Application of CCFM16 in preparation of bacterial agents, foods or medicines for relieving infantile autism and adsorbing polychlorinated biphenyl
A CCFM16, polychlorinated biphenyl technology, used in food or drug applications, in the field of bacterial agents, can solve problems such as neuronal function damage, achieve social barriers, relieve muscle rigidity and postural gait disorders, and improve stereotyped behaviors Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Bifidobacterium bifidum CCFM16 has good tolerance to simulated gastrointestinal fluid
[0045] The cryopreserved Bifidobacterium bifidum CCFM16 was inoculated in mMRS medium (MRS medium + 0.05% cysteine hydrochloride), cultured anaerobically at 37°C for 48 hours, and subcultured in mMRS medium for 2~ After 3 times, take 1 mL of the culture solution of Bifidobacterium bifidum CCFM16, mix it with 9.0 mL of pH 2.5 artificial simulated gastric juice (containing 1% pepsin, mMRS medium at pH=2.5), and culture it anaerobically at 37°C. Samples were taken at 0h, 0.5h, 1h, and 2h, and the mMRS agar medium was used to pour culture to count plate colonies, determine the number of viable bacteria and calculate their survival rate.
[0046] The survival rate is the ratio of the logarithmic value of the number of viable bacteria at the time of sampling to the logarithmic value of the number of viable bacteria at the 0th hour in the culture solution, expressed in %. Take...
Embodiment 2
[0051] Embodiment 2: Bifidobacterium bifidum CCFM16 has no toxic and side effects to wistar rats
[0052] Bifidobacterium bifidum CCFM16 cells were resuspended in 10% skim milk to make a concentration of 3.0×10 8 CFU / mL bacterial suspension. Six male wistar autistic rats with a body weight of about 200 g were taken. After one week of adaptation to the environment, the bacterial suspension of this concentration was administered orally once a day, observed for one week, and the death and body weight were recorded.
[0053] The results of these tests are listed in Table 3. These results indicate that feeding concentrations of 3.0×10 8 CFU / mL of Bifidobacterium bifidum CCFM16 had no significant impact on rats, no significant changes in body weight, and no death. The appearance of the rats showed no obvious pathological symptoms.
[0054] Table 3 Changes in body weight and death of mice
[0055]
[0056] Note: -: Rats did not die
Embodiment 3
[0057] Example 3: Bifidobacterium bifidum CCFM16 can significantly improve the autonomous exploration ability of autistic rats
[0058] (1) Breeding of young mice with autism
[0059] Healthy male and female wistar rats were bred and adapted to the environment for 1 week. They were caged together at 5:00 p.m. at a ratio of 2:1, and the vaginal plug was observed at 8:00 the next morning. If no vaginal plug was observed, a vaginal smear was performed. On the first day of pregnancy, the female mice were housed in separate cages after conception, and randomly divided into model group and control group. On the 12.5th day after pregnancy, intraperitoneal injection of sodium valproate (VPA) 600mg / kg (normal saline made into 250mg / mL solution) was given as the model group; Weaning.
[0060]
[0061] (2) Experimental grouping of autistic rats
[0062] Divided into control group, model group and intervention group.
[0063]
[0064]
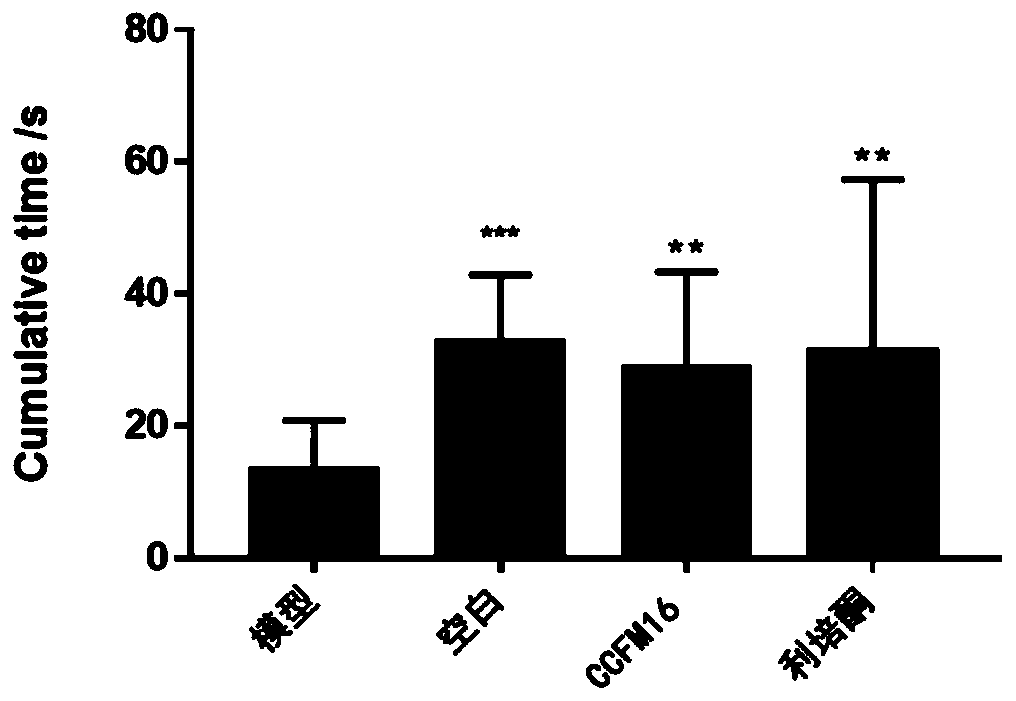
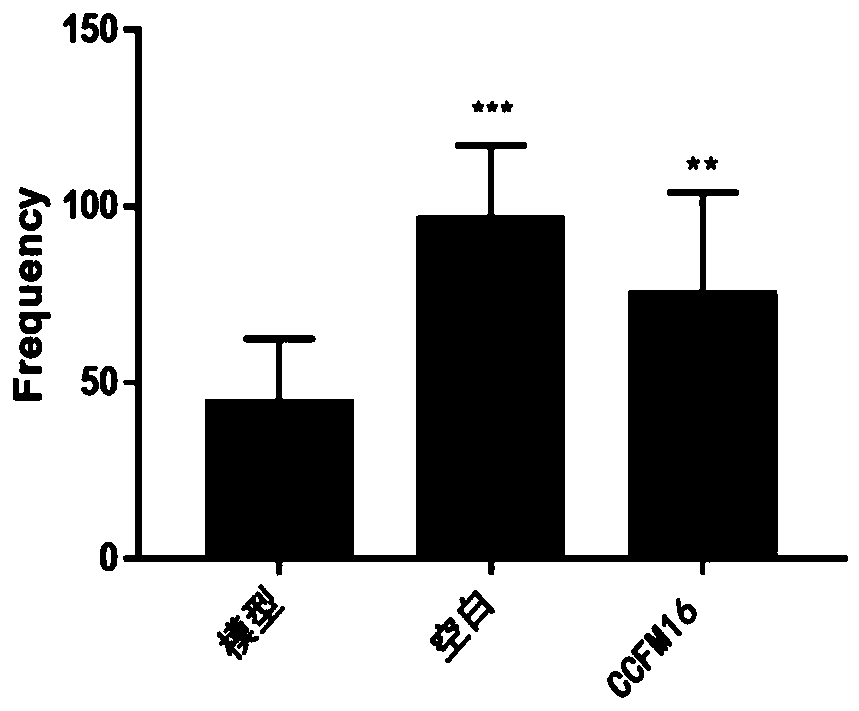
[0065] (3) Open field test of autistic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com