Compound with androgen receptor (AR) degradation activity
A compound, halogen technology, applied in the field of medicine, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0264] Compound preparation
[0265] Preparation
[0266] The preparation method of the compound of formula I of the present invention is described in more detail below, but these specific methods do not constitute any limitation to the present invention. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0267] The preparation method of the compound of formula I of the present invention provided by the present invention is as follows.
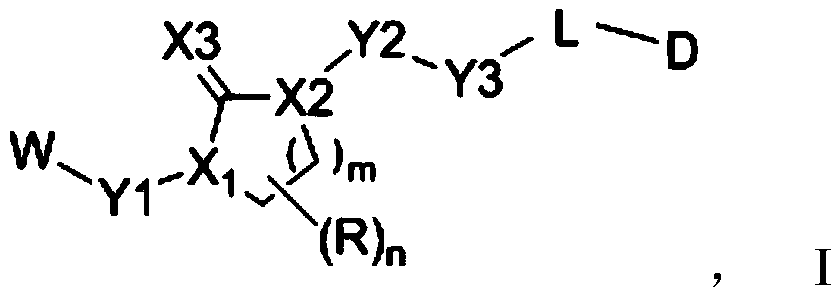
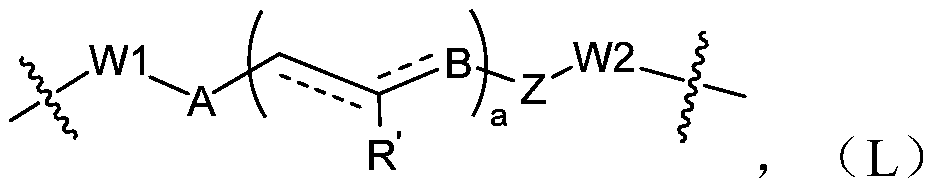
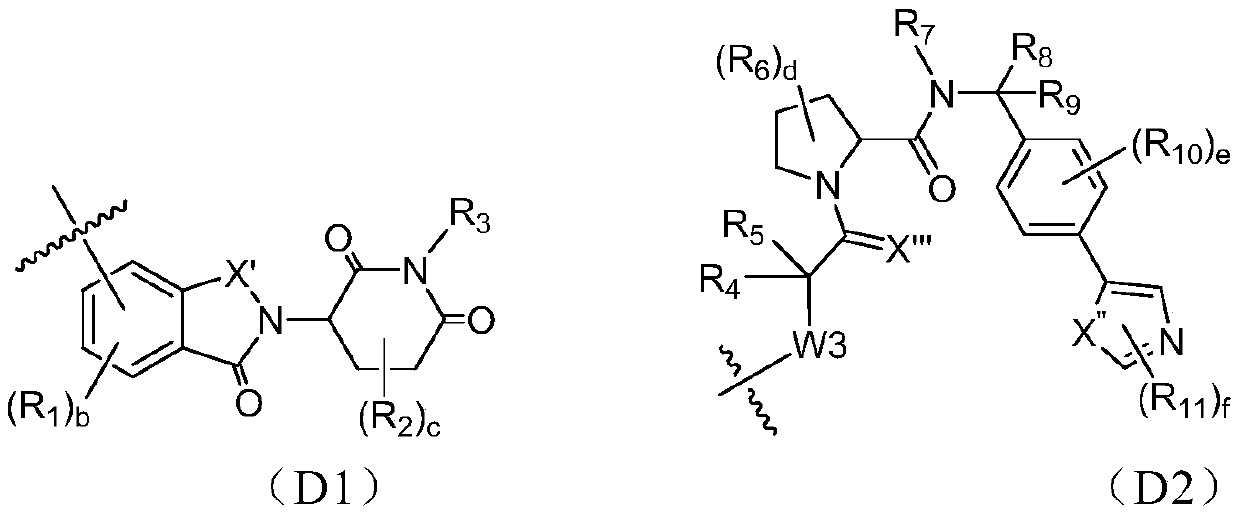
[0268] The following reaction schemes illustrate the preparation of compounds of the invention. Unless otherwise indicated, W, Y1, Y2, Y3, X1, X2, X3, R, L, D, n, m in the reaction schemes and subsequent discussions are as defined above; RE 1 、RE 2 、RE 3 、RE 4 When connecting in pairs, one end is (or can b...
Embodiment 1
[0309] The preparation of embodiment 1 compound 5
[0310]
[0311] first step
[0312] Dissolve DL-panthenolactone 1 (7.81g) in anhydrous tetrahydrofuran (200ml), add 60% sodium hydride (3.00g), stir the reaction, and after the gas release is complete, add 4-fluoro-2-(tri Fluoromethyl)benzonitrile (9.46g), react overnight at room temperature. The reaction solution was concentrated, and the residue was purified by a silica gel column to obtain 10.69 g of compound 2 as an off-white solid. Yield: 63%. MS(ESI):300.1[M+H] + .
[0313] second step
[0314] Compound 2 (2.99g) was dissolved in toluene (20ml), at room temperature, 4-(aminomethyl)phenol (1.23g) was added, the reaction solution was heated and reacted for 16 hours, the reaction solution was filtered and concentrated, and the residue was purified by a silica gel column , to obtain 2.74 g of off-white solid. The resulting solid was dissolved in pyridine (10ml), methanesulfonyl chloride (1.26g) was added dropwise ...
Embodiment 2
[0319] The preparation of embodiment 2 compound 11
[0320] first step
[0321] Compound 2 (5.98g) was dissolved in ethanol (60ml), and 2,4-dimethoxybenzylamine (10.00g) was added at room temperature, and the reaction solution was stirred at 60°C overnight. The reaction solution was concentrated by filtration, and the residue was purified by a silica gel column to obtain 6.44 g of off-white solid. The obtained solid was dissolved in pyridine (50ml), and methanesulfonyl chloride (1.89g) was added dropwise in an ice-water bath, and the stirring was continued until the raw material basically disappeared. After washing with brine, the organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The obtained residue was dissolved in anhydrous tetrahydrofuran (50ml), 60% sodium hydride (1.10g) was added in portions, and stirred overnight at room temperature. The reaction solution was quenched with saturated ammonium chloride (50ml), extracted w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com