Imidazole side chain type anion exchange membrane for fuel cells and preparation method of imidazole side chain type anion exchange membrane
An anion exchange membrane, fuel cell technology, applied in fuel cells, circuits, electrical components, etc., can solve problems such as high price and restricting the promotion and development of proton exchange membrane fuel cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
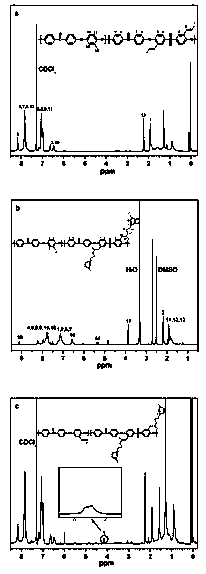
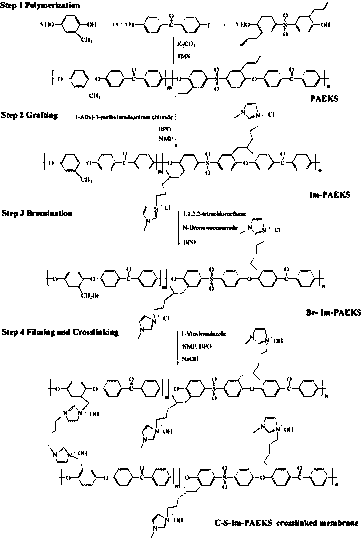
[0034] The present invention also provides a kind of preparation method of imidazole side chain type anion exchange membrane for fuel cell, comprises as follows:
[0035] Step 1: Dissolve imidazole-functionalized polyaryl ether ketone sulfone in a solvent to obtain a clear orange solution, add 1-allyl-3-methylimidazole chloride and stir for 8 hours, then add benzoyl peroxide at 80 Stirred under the condition of 3 hours for 3 hours, the material was discharged in distilled water to obtain a gray solid;
[0036] Step 2: Dissolve the gray solid obtained in the first step in tetrachloroethane, add benzoyl peroxide and N-bromosuccinimide and stir in an 80-degree oil bath for 5 hours, and discharge it in alcohol to obtain a light yellow color floc;
[0037] Step 3: dissolving the light yellow floc obtained in the second step in a solvent to obtain a clear orange solution;
[0038] Step 4: Add benzoyl peroxide and 1-vinylimidazole to the solution obtained in step 3 and stir for 12 ...
Embodiment 1
[0043] (1) Dissolve imidazole-functionalized polyaryl ether ketone sulfone in N-methylpyrrolidone (NMP) solvent (at this time, the molar ratio of methyl hydroquinone monomer to allyl bisphenol S monomer is 5:5), Then to obtain a clear orange solution, add 1-allyl-3-methylimidazole chloride and stir for 8 hours, then add benzoyl peroxide and stir for 3 hours under the condition of 80 degrees, discharge into distilled water to obtain a gray solid;
[0044] (2) Dissolve the gray solid obtained in step (1) in tetrachloroethane, add benzoyl peroxide and N-bromosuccinimide and stir in an 80-degree oil bath for 5 hours, and discharge the material in alcohol to obtain yellow floc;
[0045] (3) dissolving the yellow floc obtained in step (2) in a solvent, and then obtaining a clear orange solution;
[0046] (4) Add benzoyl peroxide and 1-vinylimidazole to the solution obtained in step (3) and stir for 12 to 24 hours to obtain a dark yellow film-forming liquid. The molar ratio of bisphe...
Embodiment 2
[0050] (1) Dissolve imidazole-functionalized polyaryl ether ketone sulfone in N-methylpyrrolidone (NMP) solvent (at this time, the molar ratio of trimethylhydroquinone monomer to allyl bisphenol S monomer is 3:7) , and then to obtain a clear orange solution, add 1-allyl-3-methylimidazole chloride and stir for 8 hours, then add benzoyl peroxide and stir for 3 hours at 80 degrees, and discharge in distilled water to obtain a gray solid ;
[0051] (2) Dissolve the gray solid obtained in step (1) in tetrachloroethane, add benzoyl peroxide and N-bromosuccinimide and stir in an 80-degree oil bath for 5 hours, and discharge the material in alcohol to obtain yellow floc;
[0052] (3) dissolving the yellow floc obtained in step (2) in a solvent, and then obtaining a clear orange solution;
[0053] (4) Add benzoyl peroxide and 1-vinylimidazole to the solution obtained in step (3) and stir for 12 to 24 hours to obtain a dark yellow film-forming liquid. The molar ratio of bisphenol S m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com