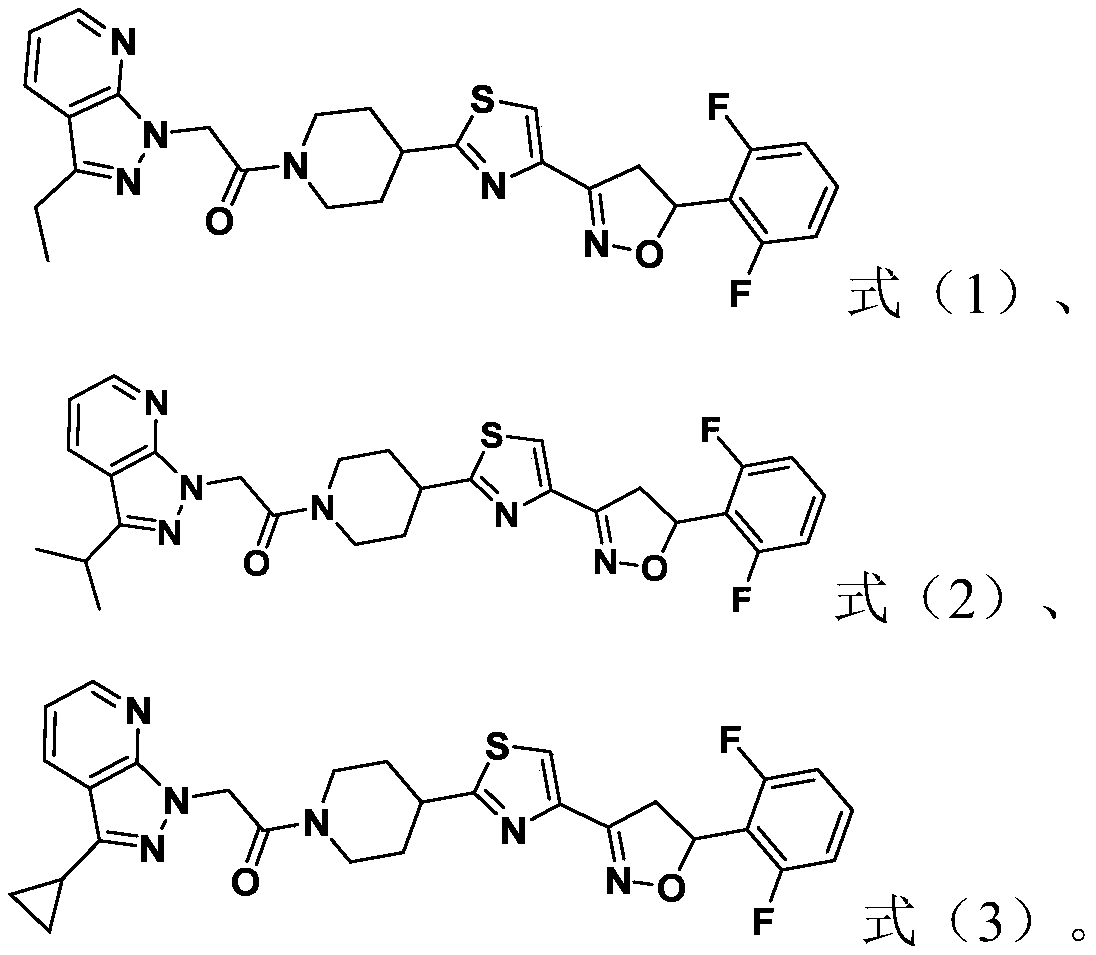
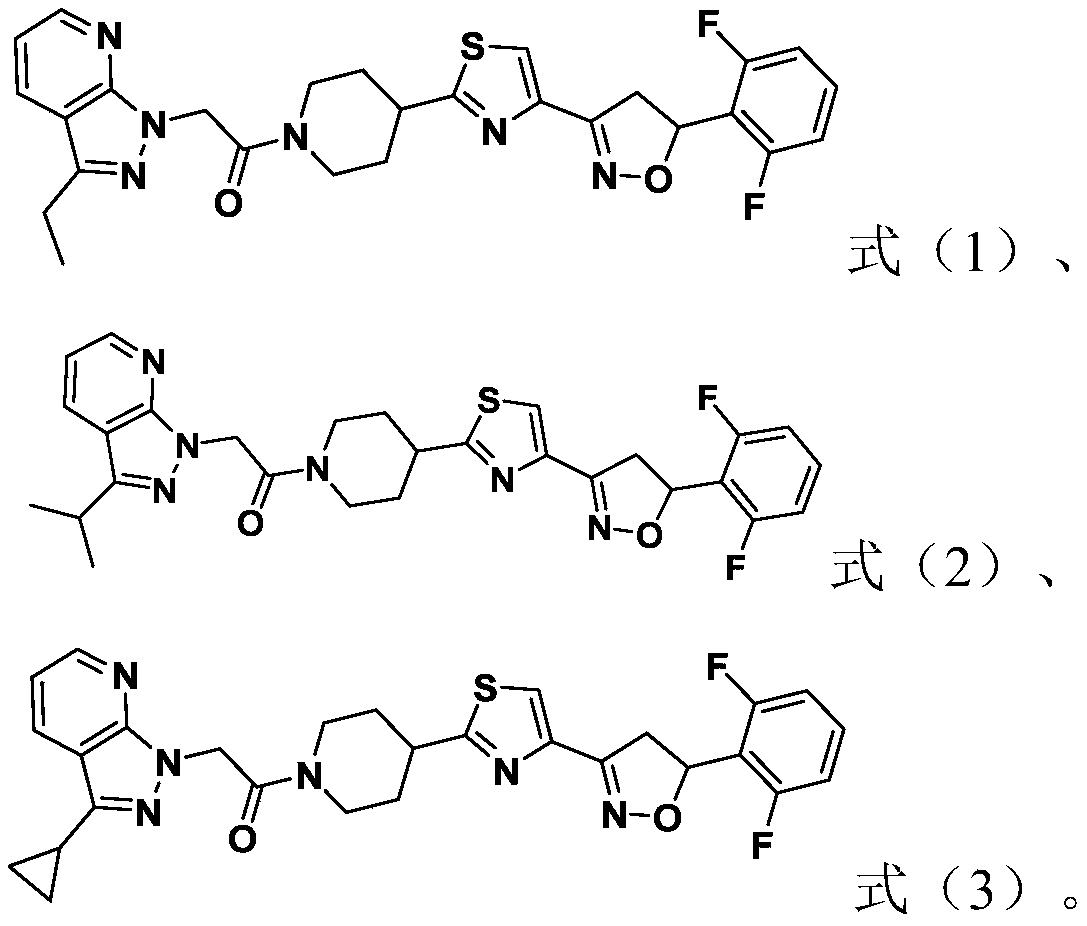
Compound with fused heterocycle structure as well as preparation method and application thereof and fungicide
A compound and fused heterocyclic ring technology, applied in the field of pesticides, fungicides and fungicides, can solve the problems of prolonged use time, irrational use, pathogenic bacteria resistance, etc., and achieve the effect of good market development prospects and excellent control effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
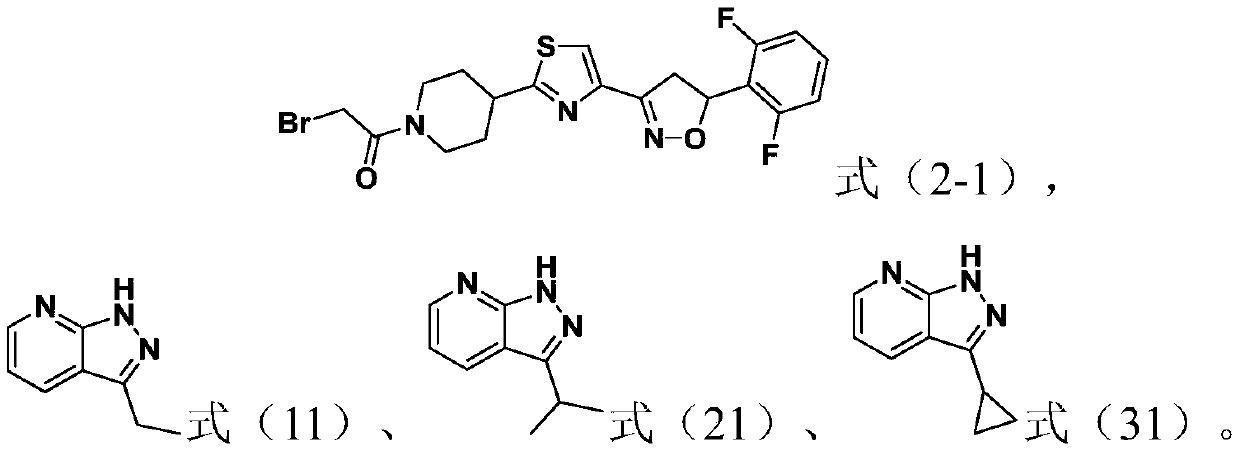
[0041] This example is used to illustrate the preparation method of the compound represented by formula (2-1).
[0042]
[0043] (1) At 25°C, add 1,3-dichloroacetone (1mol) into 1L of 2M diethyl ether hydrochloride solution, then add tert-butyl nitrite (1mol), continue the reaction at 25°C for 10h, and remove Diethyl ether, after cooling, obtain the crude product of the compound shown in formula (2-2), and wash with 1-chlorobutane, filter, collect filter cake; And the filtrate is recrystallized, filter, also obtain formula (2 The compound shown in -2) was combined to obtain the pure product of the compound shown in formula (2-2) after merging two batches.
[0044] (2) At 25°C, add sodium bicarbonate (5mol) to 1L of the compound (1mol) shown in formula (2-2) in dichloromethane solution, then add 2,6-difluorostyrene (1mol) Add slowly, continue to stir and react for 4h, after the reaction is completed, filter and remove the solvent in the filtrate to obtain the compound repre...
Embodiment 2
[0049] This example is used to illustrate the preparation method of the compound represented by formula (1).
[0050]
[0051] At 0°C, add sodium hydride (1.5mmol) to a 10mL DMF solution of the compound (1.2mmol) represented by formula (11), react at 0°C for 0.5h, and then add the compound represented by formula (2-1) Compound (1 mmol), continue to react at 25°C for 6h. After completion of the reaction, extract, wash, concentrate, and purify by column chromatography to obtain the compound shown in formula (1).
[0052] White solid, yield 89%, m.p.128-130℃, 1 H NMR (600MHz, CDCl 3 )δ8.51 (dd, J=4.8,1.6Hz,1H),8.06(dd,J=8.4,1.6Hz,1H),7.67(s,1H),7.36–7.28 (m,1H),7.10(dd ,J=8.4,4.8Hz,1H),6.92(t,J=8.4Hz,2H),6.08(dd,J=12.0,9.0Hz,1H),5.39(s,2H),4.62(d,J= ( m, 2H), 3.02(q, J=7.8Hz, 2H), 2.85(t, J=12.6Hz, 1H), 2.23(d, J=13.8Hz, 1H), 2.16(d, J=13.8Hz, 1H), 1.90–1.75(m, 2H), 1.41(t, J=7.8Hz, 3H). 13 CNMR (151MHz, CDCl 3 )δ174.36, 165.09, 162.04(d, J=5.1Hz), 160.37(d, J=5.1Hz), ...
Embodiment 3
[0055] This example is used to illustrate the preparation method of the compound represented by formula (2).
[0056]
[0057] At 0°C, add sodium hydride (1.5mmol) into a 10mL DMF solution of the compound (1.2mmol) represented by formula (21), react at 0°C for 0.5h, and then add the compound represented by formula (2-1) Compound (1 mmol), continue to react at 25°C for 6h. After completion of the reaction, extract, wash, concentrate, and purify by column chromatography to obtain the compound shown in formula (2).
[0058] White solid, yield 79%, m.p.165-166℃, 1 H NMR (600MHz, DMSO-d 6 )δ 8.48(s,1H),8.31(d,J=7.8Hz,1H),8.04(s,1H),7.55–7.45(m,1H),7.27–7.07(m,3H),6.05–5.95( m,1H),5.46(d,J=16.8Hz,1H),5.36(d,J=16.8Hz,1H),4.35(d,J=13.2Hz,1H),4.13(d,J=13.2Hz, 1H),3.91(t,J=14.4Hz, 1H),3.54(dd,J=17.4,8.4Hz,1H),3.36–3.24(m,3H),2.81(t,J=12.6Hz, 1H), 2.10(t, J=16.2Hz, 2H), 1.78(d, J=14.4Hz, 1H), 1.57(t, J=12.6Hz, 1H), 1.36(d, J=6.6Hz, 6H). 13 C NMR (101MHz, DMSO-d 6 )δ175.08,165.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com