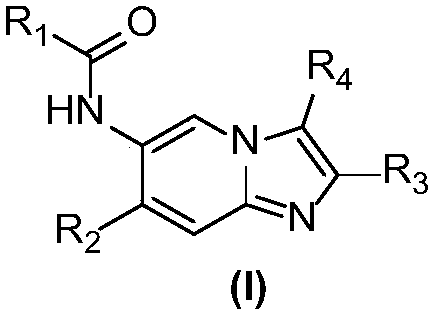
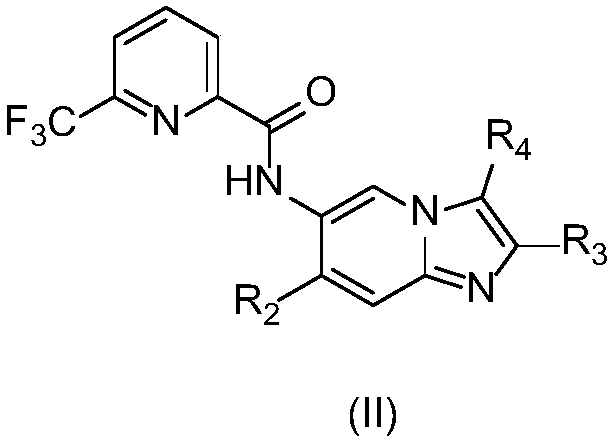
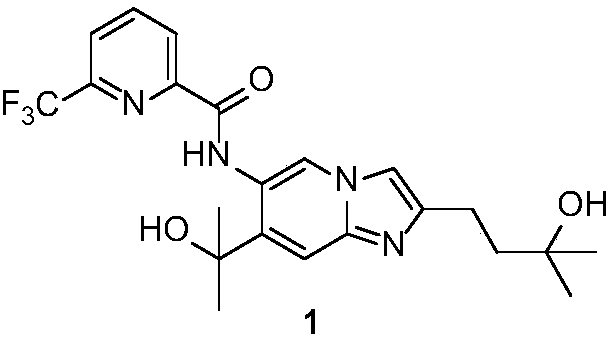
Imidazopyridine derivative, preparation method and medical uses thereof
A pharmacy and compound technology, applied in the fields of imidazopyridine derivatives and their preparation and their use in medicine, can solve the problems of unsatisfactory effectiveness, safety or applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] N-(2-(3-hydroxy-3-methylbutyl)-7-(2-hydroxypropan-2-yl)imidazol[1,2-a]pyridin-6-yl)-6-(trifluoro Methyl)pyridine-2-carboxamide
[0055]
[0056] The first step is to synthesize 2-amino-5-nitroisonicotinic acid methyl ester
[0057]
[0058] At 0°C, slowly add 10g of 2-aminoisonicotinic acid methyl ester into 100mL of concentrated sulfuric acid with mechanical stirring, stir until dissolved and then lower the temperature to -10°C, slowly drop the mixed acid prepared by 10mL of concentrated nitric acid and 6mL of concentrated sulfuric acid Add it to the reaction (dropping for about 30 minutes), after the dropwise addition is complete, control the reaction temperature below -5°C and continue stirring overnight. After the reaction was completed, the reaction solution was added to a large amount of ice, the pH was adjusted to neutral to slightly alkaline with concentrated ammonia water, the solid was precipitated by stirring, filtered with suction, and vacuum-dried at...
Embodiment 2
[0077] N-(7-(2-Hydroxypropan-2-yl)-2-(piperidin-4-yl)imidazo[1,2-a]pyridin-6-yl)-6-(trifluoromethyl)pyridine -2-Carboxamide
[0078]
[0079] The first step is to synthesize 2-(1-(tert-butoxycarbonyl)piperidin-4-yl)-6-nitroimidazo[1,2-a]pyridine-7-carboxylic acid methyl ester
[0080]
[0081] Weigh 1b (1g, 5mmol), 2a (2eq, 3.1g) and magnesium oxide (2eq, 0.4g), add THF (50mL), seal the tube at 100°C for 24h, cool the reaction solution to room temperature, suction filter, and wash with THF , the filtrate was spin-dried, purified by silica gel column, and eluted with Hex / THF to obtain 2b (1.13g, 56%).
[0082] The second step is to synthesize 6-amino-2-(1-(tert-butoxycarbonyl)piperidin-4-yl)imidazo[1,2-a]pyridine-7-carboxylic acid methyl ester
[0083]
[0084] Weigh 2b (1.13g, 2.8mmol), iron powder (1.57g, 10eq) and ammonium chloride (0.45g, 3eq), add ethanol / water (100mL, volume ratio 4 / 1), and react at 90°C until raw material 2b The reaction was complete, filtere...
Embodiment 3
[0098] N-(7-(2-hydroxypropan-2-yl)-2-(1-methylpiperidin-4-yl)imidazo[1,2-a]pyridin-6-yl)-6-(trifluoro Methyl)pyridine-2-carboxamide
[0099]
[0100] Weigh 2 (45mg, 0.1mmol) and dissolve it in DMF (5mL), add potassium carbonate (27mg, 2eq), react at room temperature for 30min, add iodomethane (21mg, 1.5eq), TLC detection until the reaction of raw material 2 is complete, add saturated salt Washed with water (20 mL), added 100 mL of ethyl acetate for extraction, separated the organic layer, dried over anhydrous sodium sulfate, filtered, concentrated, purified on a silica gel column, and eluted with dichloromethane / methanol to obtain compound 3 (27 mg, 60%). 1 H NMR (400MHz, CDCl 3 )δδ12.44(s,1H),9.78(s,1H),8.43(d,J=7.8Hz,1H),8.14(t,J=7.8Hz,1H),7.90-7.80(m,2H), 7.44(s,1H),4.12(br,1H),3.90-3.80(m,2H),2.95-2.84(m,2H),2.81(s,3H),2.35-2.25(m,2H),1.90- 1.83(m,2H),1.77(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com