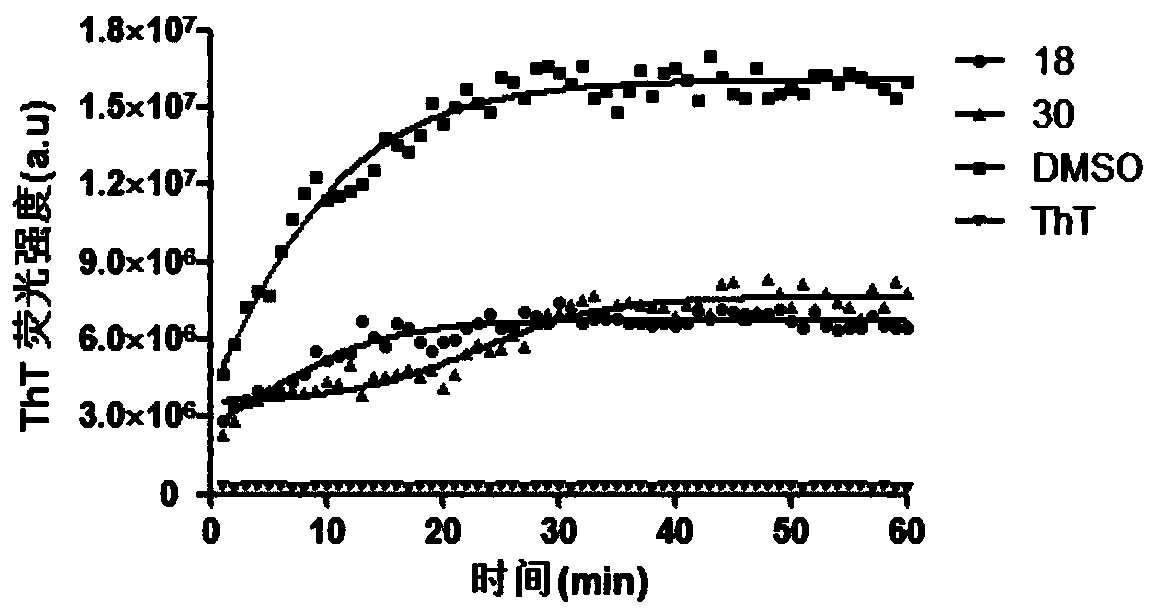
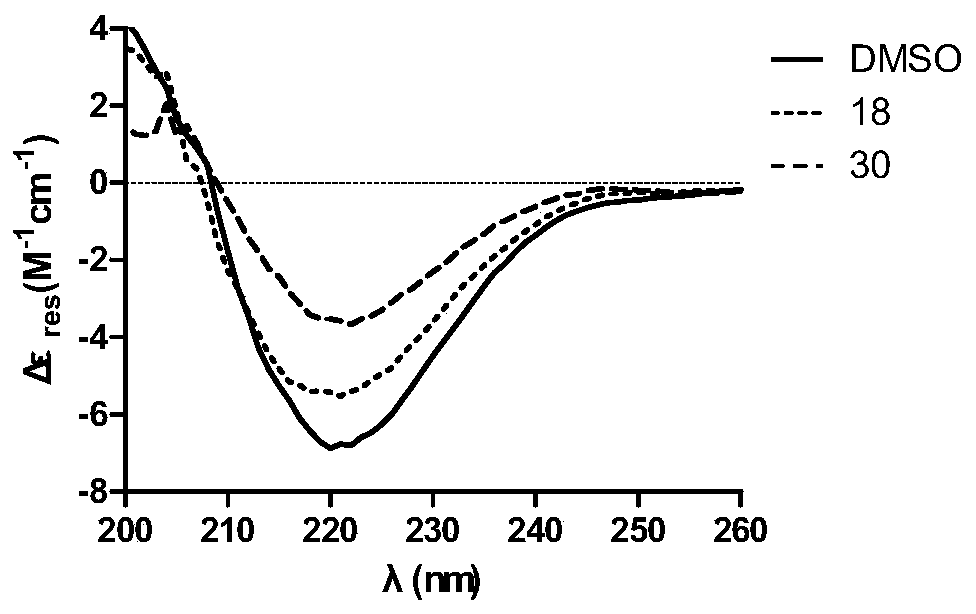
Usnic acid derivatives, and preparation method and applications thereof in Alzheimer's disease drugs
A derivative, usnic acid technology, applied in the field of structural modification of natural compounds, can solve problems such as difficulty in simplification of natural products, difficulty in the source and de novo synthesis of natural products, lack of cytotoxicity or research on the improvement effect of animal models, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Compound (4) of Example 1: (R,E)-6-acetyl-7,9-dihydroxy-2-(1-((5-hydroxypentyl)amino)ethylene)-8,9b- Preparation of dimethyldibenzo[b,d]furan-1,3(2H,9bH)-dione.
[0079] 100 mg (+)-usnicic acid (0.290 mmol, 1.0 eq) was suspended in 5 mL of absolute ethanol, and 0.033 mL of 5-amino-1-pentanol (0.320 mmol, 1.1 eq) was slowly added dropwise while stirring. After the dropwise addition, the reaction was carried out at 80°C under the protection of argon for 4 h until the completion of the reaction was monitored by a spot plate. After the solution was cooled to room temperature, it was acidified with 1N hydrochloric acid solution, the mixture was extracted three times with ethyl acetate and the organic phases were combined. The organic phase was washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, concentrated, mixed and passed through a silica gel column, and the petroleum ether / ethyl acetate product system was obtained to obtain t...
Embodiment 2
[0080]Example 2 compound (5): (R, E)-6-acetyl-2-(1-((5-chloro-2-hydroxyphenyl)amino)ethylene)-7,9-dihydroxy- Preparation of 8,9b-dimethyldibenzo[b,d]furan-1,3(2H,9bH)-dione.
[0081] Preparation process is with example 1. The starting material was replaced with 4-chloro-2-aminophenol. The yield is about 89%, yellow solid. m.p.154-155℃. 1 H NMR (500MHz, DMSO-d 6 )δ14.53(bs,1H),13.40(s,1H),12.02(s,1H),10.62(s,1H),7.47(d,J=3.5Hz,1H),7.30(dd,J=8.5 ,3.5Hz,1H),7.03(d,J=8.5Hz,1H),6.00(s,1H),2.66(s,3H),2.52(s,3H),1.99(s,3H),1.71(s ,3H). 13 C NMR (126MHz, DMSO-d 6 )δ201.43,198.58,189.99,174.98,174.16,163.05,157.97,156.18,151.19,129.59,127.06,124.72,122.92,118.24,106.98,105.51,103.00,102.73,101.46,57.22,32.09,31.53,20.79,7.99.
Embodiment 3
[0082] Example 3 compound (6): (R, E)-6-acetyl-2-(1-((4-chloro-2-hydroxyphenyl)amino)ethylene)-7,9-dihydroxy- Preparation of 8,9b-dimethyldibenzo[b,d]furan-1,3(2H,9bH)-dione.
[0083] Preparation process is with example 1. The starting material was replaced with 2-amino-5-chlorophenol. The yield is about 84%, yellow solid. m.p.147-149℃. 1 H NMR (500MHz, DMSO-d 6 )δ14.52(bs,1H),13.40(s,1H),12.03(s,1H),10.87(s,1H),7.36(d,J=8.5Hz,1H),7.05(d,J=2.3 Hz,1H),6.98(dd,J=8.5,2.3Hz,1H),5.98(s,1H),2.66(s,3H),2.51(s,3H),1.98(s,3H),1.71(s ,3H). 13 C NMR (126MHz, DMSO-d 6 )δ201.40,198.51,189.99,174.83,174.13,163.04,157.97,156.17,152.99,133.43,128.74,122.84,119.72,116.65,106.97,105.51,103.02,102.72,101.44,57.19,32.11,31.52,20.81,7.99.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com