Synthesis method and applications of N heteroatom polysubstituted benzoquaternary cycloketone
A synthesis method and multi-substitution technology, which is applied in the field of synthesis of N-heteroatom multi-substituted benzotetracyclic ketones, can solve problems such as the difficulty of benzocyclobutanone, and achieve high applicability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
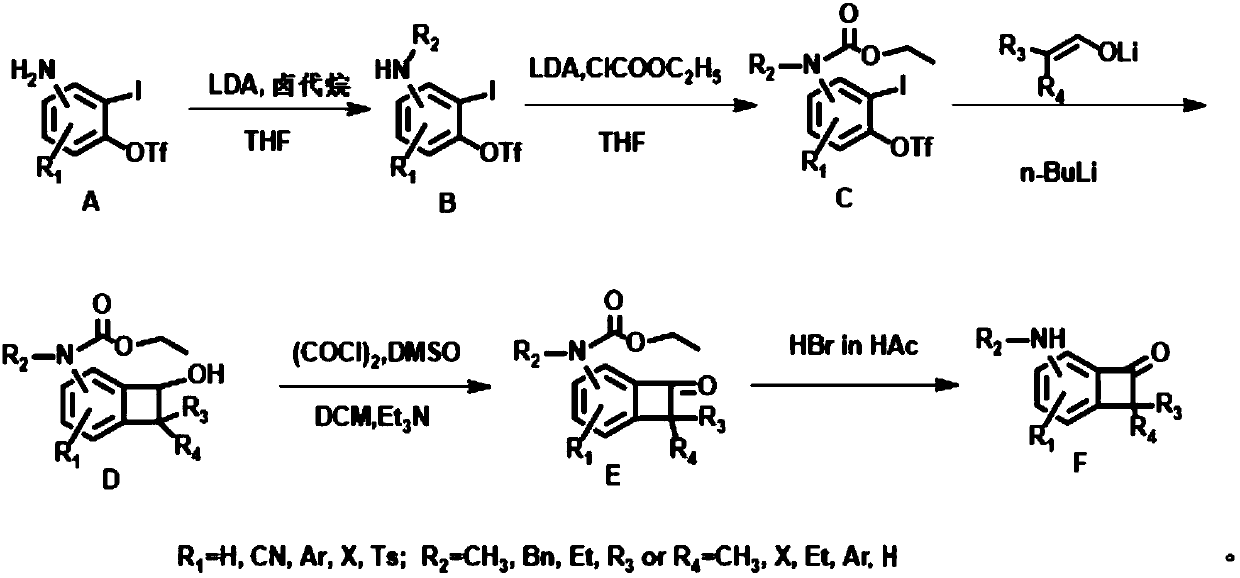
Embodiment 1
[0032] first step:
[0033]
[0034] Three THF solutions of compound A were prepared, and commercially available LDA (1.08 mmol, 0.54 mL, 2M hexane / THF solution) was added to a solution of compound A (1 mmol) in THF (2 mL) at -78 ° C, and the reactant Stir at this temperature for 20 minutes, add iodomethane, iodoethane and benzyl bromide (63uL) to the three solutions respectively, then continue to stir at room temperature for 1 hour, after the reaction is completed, wash with NH 4 Quenched with aqueous Cl. Extracted with ethyl acetate, washed with brine, MgSO 4 Drying, concentration and purification by silica gel chromatography afforded compound B1 (R 2 =CH 3 ), B2(R 2 =Et), B3(R 2 =Bn), the yield was 80-91%.
[0035] B1:R f =0.55, (PE:EA=5:1), 1 H NMR (500MHz, Chloroform-d) δ7.27 (td, J = 8.2, 1.2Hz, 1H), 6.73–6.60 (m, 1H), 6.51 (dd, J = 8.4, 1.4Hz, 1H), 2.94 ( d,J=1.3Hz,3H). 13 C NMR (126MHz, Chloroform-d) δ150.83, 150.76, 130.48, 120.15, 117.60, 109.59, 109.58,...
Embodiment 2
[0063]
[0064] Concrete experimental operation is with embodiment one, and the product that makes is as follows:
[0065] F4:R f =0.7, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.40(s,1H),7.17(s,1H),3.94(s,2H),3.36(s,3H).
[0066] F5:R f =0.6, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.24(s,1H),7.01(s,1H),3.84(s,2H),3.26(s,3H).
[0067] F6:R f =0.75, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.45(s,1H),7.23(s,1H),3.9(s,2H),3.36(s,3H).
[0068] F7:R f =0.7, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.38(s,1H),7.15(s,1H),3.92(s,2H),3.33(s,3H).
Embodiment 3
[0070]
[0071] Concrete experimental operation is with embodiment one, and the product that makes is as follows:
[0072] F8:R f =0.52, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) 1 H NMR (400MHz, Chloroform-d) δ7.40(d, J=7.26Hz, 1H), 7.14(d, J=7.26Hz, 1H), 6.88(s, 1H), 3.82(s, 2H), 3.09 (s,3H).
[0073] F9:R f =0.57, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) 1 H NMR (400MHz, Chloroform-d) δ7.42 (d, J = 7.26Hz, 1H), 7.16 (d, J = 7.26Hz, 1H), 6.88 (s, 1H), 3.82 (s, 2H), 3.09 (s,3H).
[0074] F10:R f =0.6, (PE:EA=5:1) 1 H NMR (400MHz, Chloroform-d) δ7.32(d, J=4.8Hz, 4H), 7.30–7.26(m, 1H), 7.21(t, J=4.8Hz, 1H), 6.7(d, J= 7.0Hz, 1H), 6.45(d, J=8.4Hz, 1H), 4.64(s, 2H), 3.80(s, 2H).
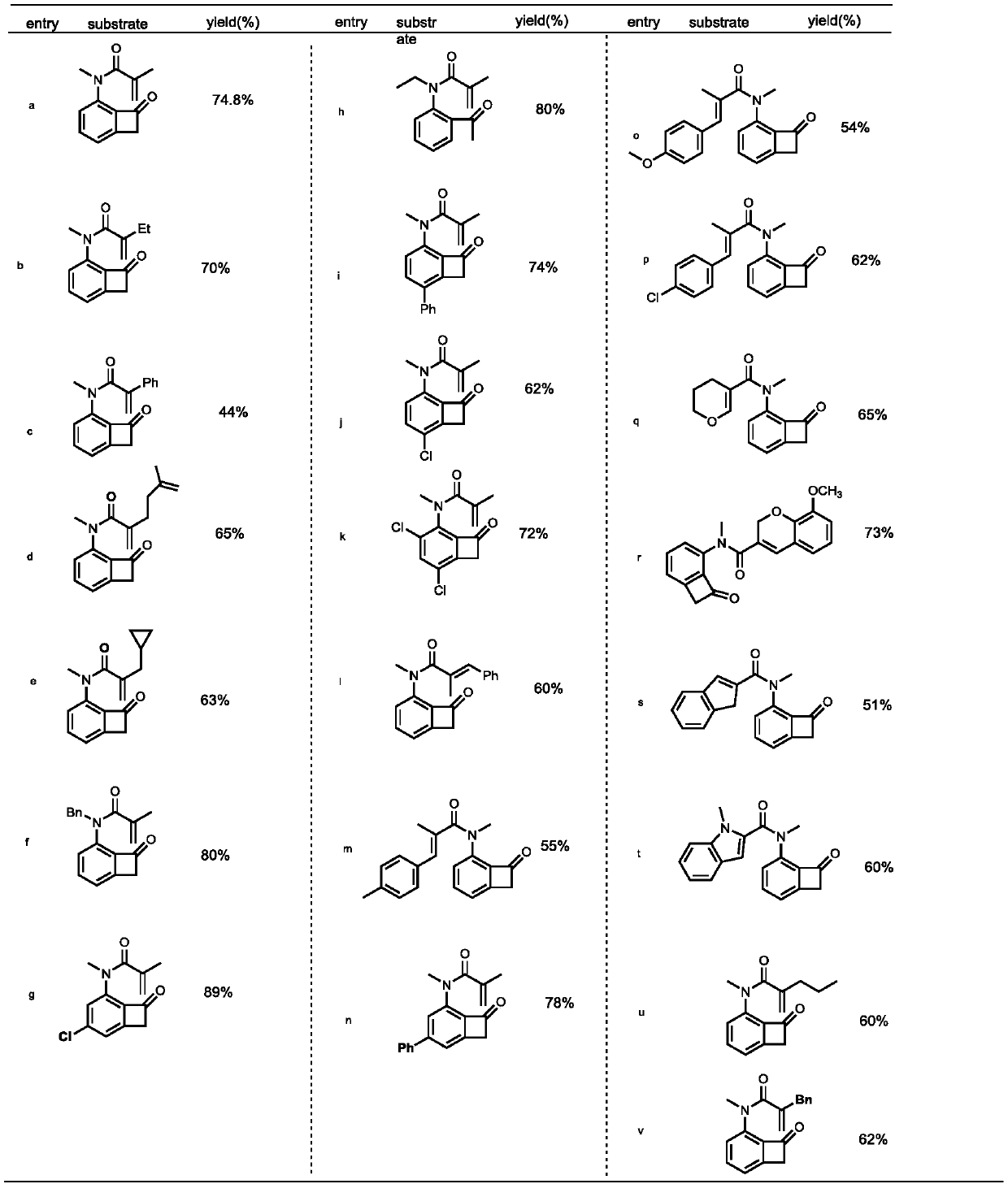
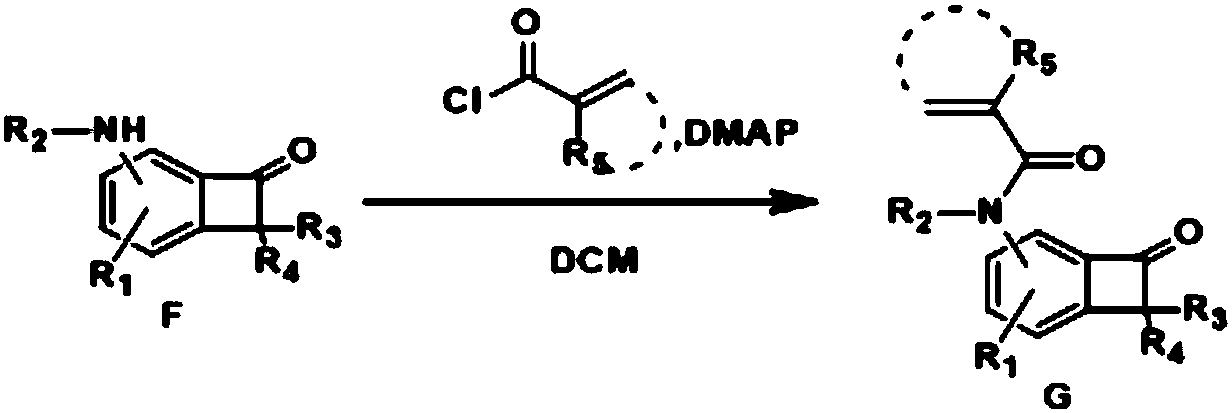
[0075] Part II: Application of N heteroatom multi-substituted benzotetracyclic ketones
[0076] The first step, the preparation of acid chloride:
[0077] figure 1 Among them, the preparation process of the required acid chlorides for Gb, Gd, Ge, Gq, Gt is consistent, as follows:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com