A class of chiral beta-hydroxyamide compounds, preparation method and applications thereof,
A technology for hydroxyamides and compounds is applied in the field of chiral beta-hydroxyamide compounds and their preparation, and can solve the problems of low yield, complicated preparation of raw materials, low stereoselectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
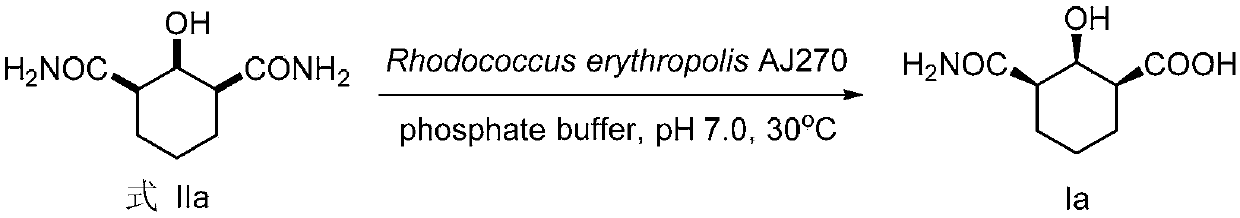
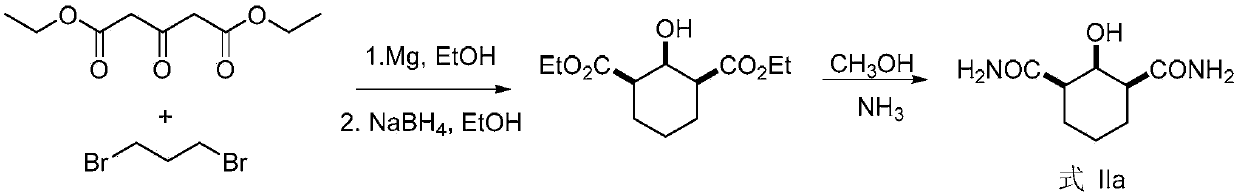
[0072] Embodiment 1, chiral amide carboxylic acid compound Ia shown in preparation formula I-1 (R 1 is -COOH, n is 3)
[0073] according to figure 1 Shown reaction equation prepares chiral amide carboxylic acid compound Ia (R 1 is -COOH, n is 3),
[0074] The specific implementation method is:
[0075] Get the Rhodococcus erythropolis AJ270 thalline of 2 grams wet weight (bacterial activity is: 1 * 10 7 -1×10 9 CFU / g), thawed at 30°C for 30 minutes, washed the bacteria with a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH 7.0, 50ml) into an Erlenmeyer flat-bottomed flask with screw top, dispersed Shake well and place in a shaker for 30 minutes at 30°C for activation, then add 1 mmol (186 mg) of the compound represented by formula IIa at one time, put it in a shaker at 30°C and 200 rpm for catalytic hydrolysis. Whole reaction TLC monitors, stops reaction after reacting 12h, and gained reaction liquid is removed thalline by o...
Embodiment 2
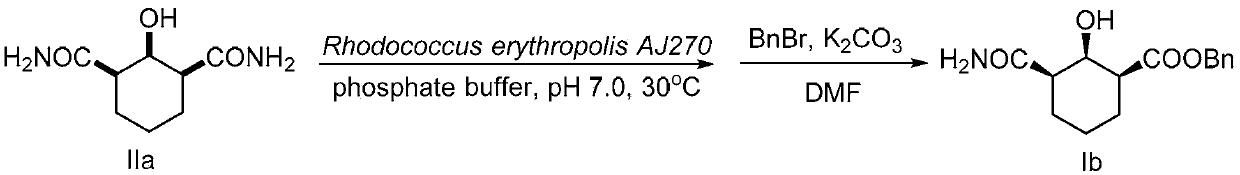
[0085] Embodiment 2, chiral amide carboxylate compound Ib shown in preparation formula I-2 (R 1 is-COOBn, n is 3)
[0086] according to image 3 Shown reaction equation prepares chiral amide carboxylic acid compound I b (R 1 is -COOBn, n is 3),
[0087] The specific implementation method is:
[0088] Take 2 grams of Rhodococcus erythropolis AJ270 bacteria in wet weight, thaw at 30°C for 30 minutes, wash the bacteria into the threaded port with a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH 7.0, 50ml) In a Erlenmeyer flat-bottomed flask, disperse and shake well, put it in a shaker for activation at 30°C for 30 minutes, then add 1 mmol (186 mg) of the compound represented by formula IIa at one time, put it in a shaker at 30°C, and carry out at 200rpm Catalyzes the hydrolysis reaction. The entire reaction was monitored by TLC, and the reaction was stopped after 12 hours of reaction. The resulting reaction solution was filtere...
Embodiment 3
[0096] Embodiment 3, the chiral amide carboxylate compound Ic shown in preparation formula I-2 (R 1 for-COOCH 2 C 6 h 4 Br, n is 3)
[0097] according to Figure 4 Shown reaction equation prepares chiral amide carboxylic acid compound Ic (R 1 for-COOCH 2 C 6 h 4 Br, n is 3),
[0098] The specific implementation method is: take 2 grams of Rhodococcus erythropolis AJ270 thallus with wet weight, thaw it for 30 minutes at 30°C, and thaw the bacteria with a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH7.0, 50ml). Wash the body into an Erlenmeyer flat-bottomed flask with a threaded opening, disperse and shake it evenly, and put it in a shaker for 30 minutes at 30°C, then add 1 mmol (186 mg) of the compound shown in Formula IIa at one time, and put it in a shaker for 30 minutes. The catalytic hydrolysis reaction is carried out at 200 rpm. The entire reaction was monitored by TLC, and the reaction was stopped after 12 hours of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com