A kind of Usnea esterified derivative and its synthetic method
A technology of usnic acid ester and synthesis method, which is applied in the directions of organic chemistry, antibacterial drugs, etc., can solve the problems such as the synthesis method of usnic acid monoester derivatives that have not been found in research, and achieve the effect of high antibacterial properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides a method for synthesizing the usnea esterified derivatives. Synthesizing the above-mentioned usnea esterified derivatives specifically includes the following steps:
[0053] Step 1: Put usnic acid in distilled water, stir in an ice-water bath, add dropwise an alkali solution, usually NaOH solution, until the usnic acid dissolves;
[0054] Wherein, the mass volume ratio of the usnic acid to the distilled water is 30~800mg / ml;
[0055] Step 2: Continue to stir for 10-100 minutes, add the corresponding acid chloride organic solution, and keep the reaction under ice-water bath conditions until the reaction equilibrium is reached;
[0056] Wherein, the mol ratio of usnic acid and acid chloride is 1:5~1:7, preferably 1:6, adopts thin-layer chromatography to monitor whether to reach reaction balance;
[0057] Step 3: Use an organic solvent to extract to neutrality, dry, and recover the solvent under reduced pressure to obtain a crude produc...
Embodiment 1
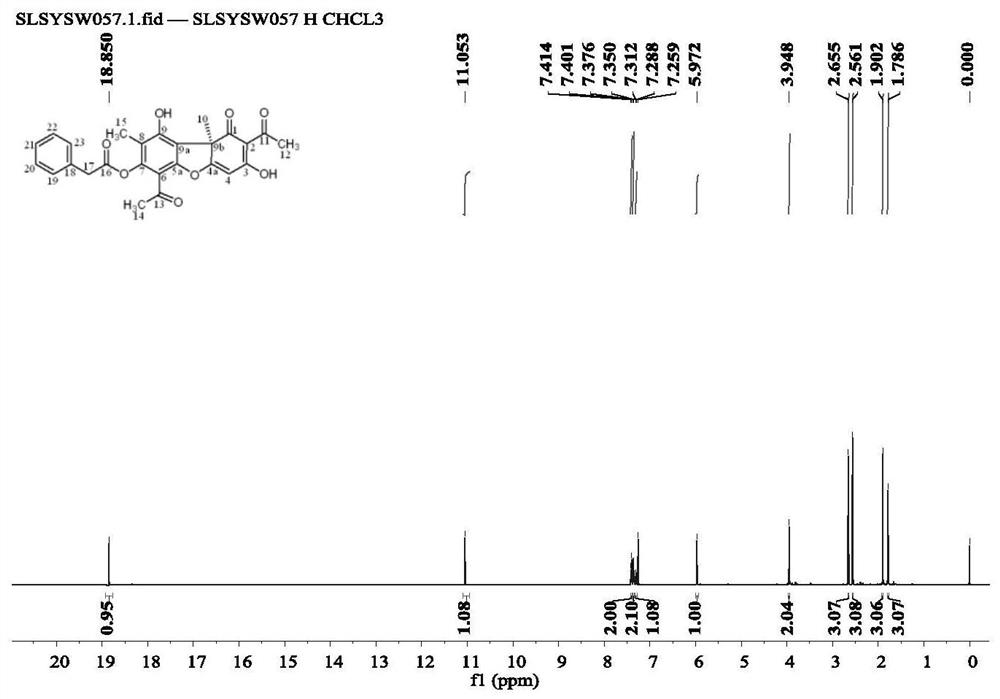
[0059] Weigh 500 mg of usnic acid into a reaction flask, add 2-3 ml of distilled water, stir in an ice-water bath, and add a few drops of 0.1-0.3 g / ml NaOH solution. Stirring was continued for 10-20min, anhydrous acetone dilution of phenylacetyl chloride (1.54ml) was added, and the reaction was maintained in an ice-water bath for 30-80min, and the reaction progress was monitored by thin-layer chromatography until the reaction reached equilibrium. Extracted with dichloromethane to neutrality, dried over anhydrous sodium sulfate, and recovered the solvent under reduced pressure to obtain a crude product, which was separated by silica gel column chromatography to obtain 404 mg of the product (compound 1), a yellow oil, the yield was 60.1%, and the structure was
[0060]
[0061] Proton NMR spectrum analysis: 1 H NMR (CDCl 3 ,600MHz)δ:1.79(s,3H),1.90(s,3H),2.56(s,3H),2.66(s,3H),3.95(s,2H),5.97(s,1H),7.29-7.31 (m,1H),7.35-7.38(m,2H),7.40-7.41(m,2H),11.05(s,1H),18.85(s,1H). fi...
Embodiment 2
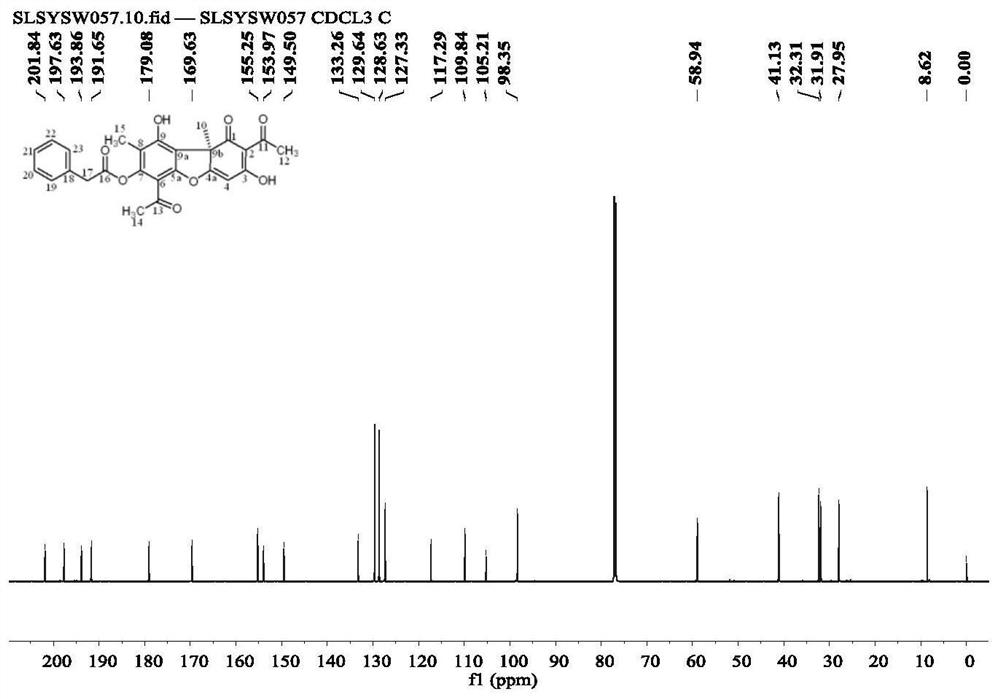
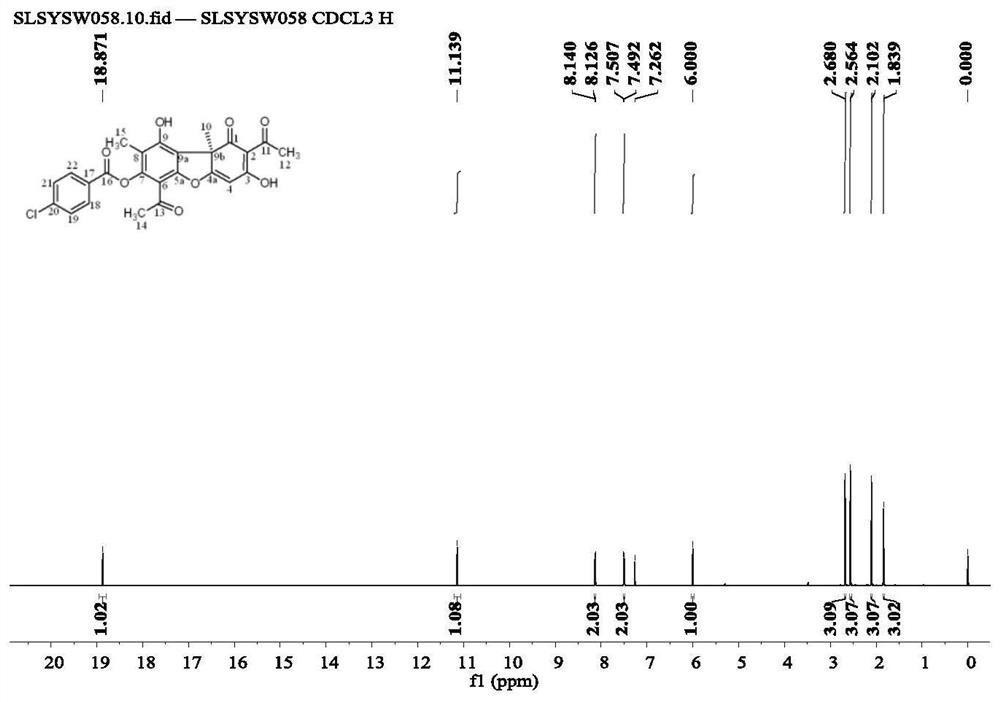
[0064] Weigh 500 mg of usnic acid into a reaction flask, add 2-3 ml of distilled water, stir in an ice-water bath, and add a few drops of 0.1-0.3 g / ml NaOH solution. Continue to stir for 10-20min, add p-chlorobenzoyl chloride (1.48ml) anhydrous acetone diluent, keep the reaction in an ice-water bath for 30-80min, and monitor the reaction progress by thin-layer chromatography until the reaction reaches equilibrium. Extracted with dichloromethane to neutrality, dried over anhydrous sodium sulfate, recovered the solvent under reduced pressure to obtain a crude product, separated by silica gel column chromatography, and further separated by Sephadex column to obtain 387.6 mg of the product (compound 2). rate 55.3%, the structure is
[0065]
[0066] Proton NMR spectrum analysis: 1 H NMR (CDCl 3 ,600MHz)δ:1.84(s,3H),2.10(s,3H),2.56(s,3H),2.68(s,3H),6.00(s,1H),7.50(d,J=8.58Hz,2H ), 8.13(d, J=8.52Hz, 2H), 11.14(s, 1H), 18.87(s, 1H). Such as image 3 shown
[0067] Carbon NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com