Medicinal use of (3,4-dihydroxyl-5-nitrophenyl)-(4-methylphenyl)ketone
A technology of nitrophenyl and methylphenyl, applied in the field of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
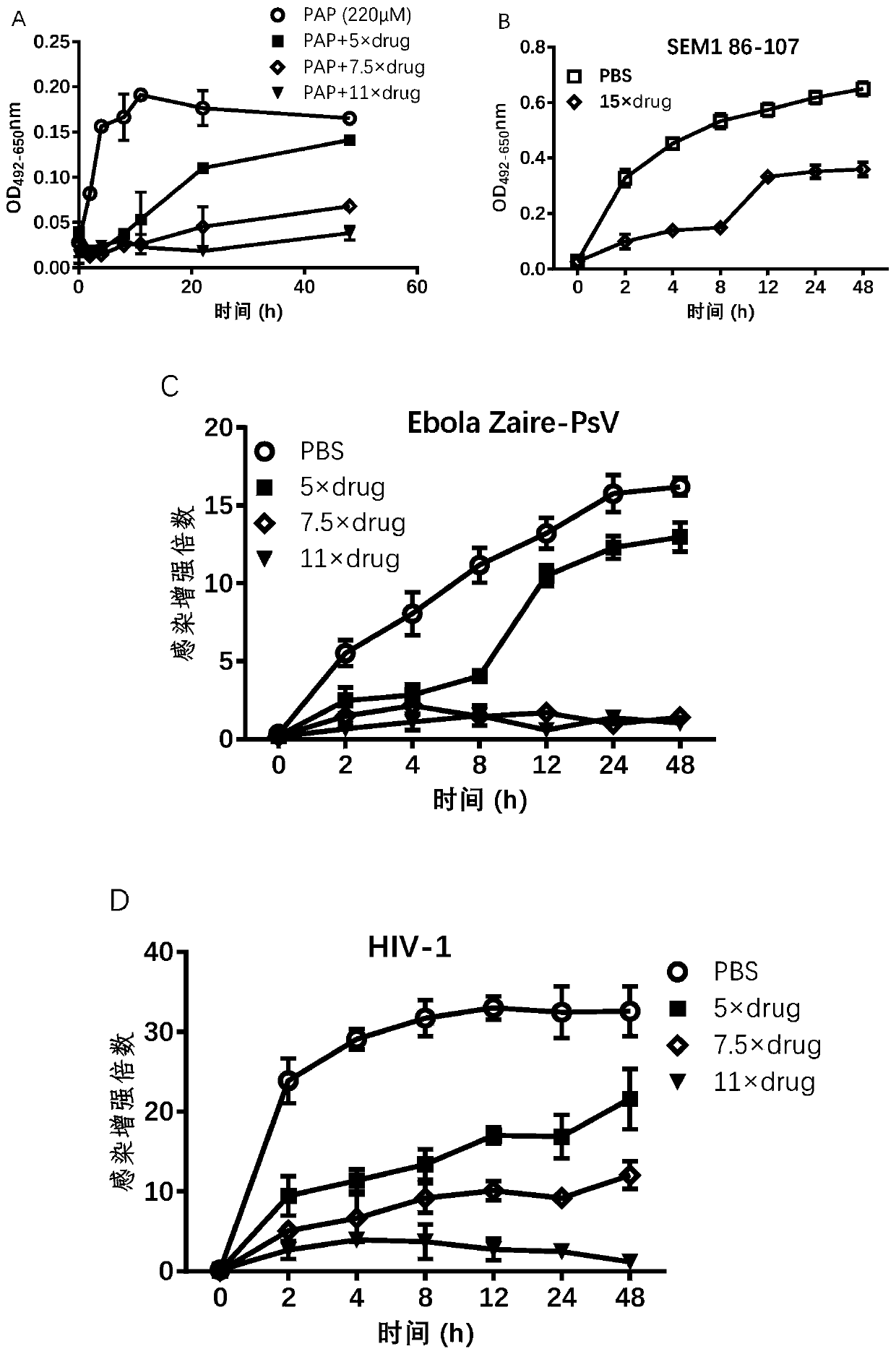
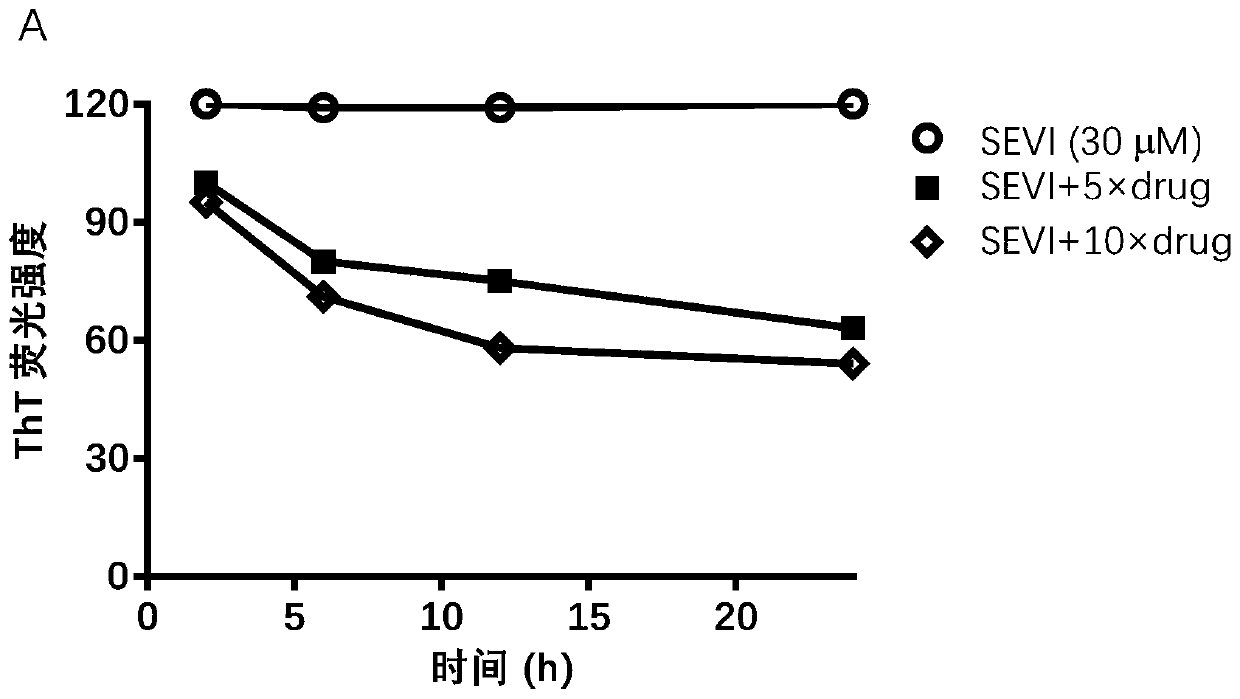
[0033] In the following in vitro experiments, (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone inhibits the formation of SEVI, inhibits the binding of SEVI to virus particles, and interrupts the fiber morphology, thereby antagonizing the promotion of SEVI. Virus infectivity was verified.
[0034] Effect of (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone on the formation of SEVI
[0035] Congo red staining: peptide PAP 248-286(220μM) mixed with various concentrations of (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone (5×, 7.5×, 11×) or PBS, placed at 37 React at 1400rpm in a shaker at ℃, take out 10μL samples at different time points (2h, 4h, 8h, 12h, 24h, 48h) and mix with 90μL Congo red solution (Sigma), react in the dark for 15min, centrifuge at 12000rpm, remove the supernatant , the precipitate was dissolved in 50 μL DMSO, and the absorbance value at 490 nm was measured, and the reference wavelength was 650 nm.
[0036] Virus infection enhancement ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com