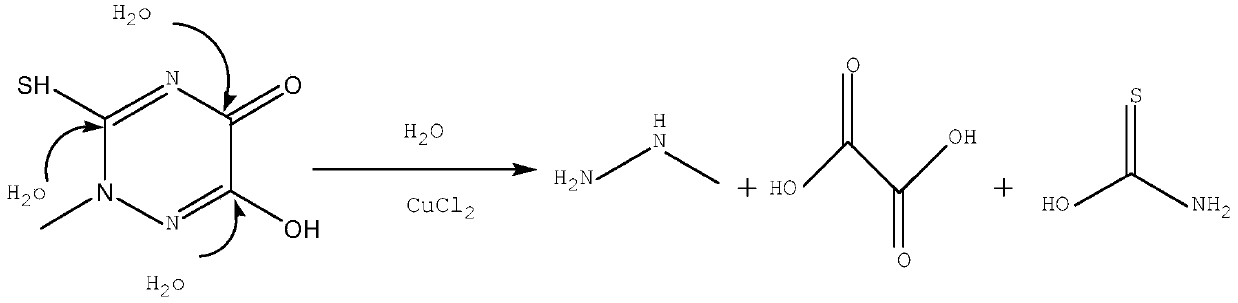
Method for decomposing triazine ring residues to recover methylhydrazine
A technology of triazine ring and methylhydrazine, which is applied in the field of recovery and utilization of by-products of pharmaceutical intermediates, can solve problems such as difficult to handle, large amount of by-products, and low product yield, and achieve the effect of low cost and easy processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 30g of triazine ring residue, 300g of water, and 2g of copper chloride to the reaction flask, start stirring, raise the temperature to 130°C and keep it warm for 2 hours, add 7.5g of caustic soda after extracting the distillate, and extract methylhydrazine after distillation of aqueous solution.
Embodiment 2
[0022] Add 30 g of triazine ring residue, 400 g of water, and 6 g of copper chloride to the reaction flask, start stirring, raise the temperature to 110° C. and keep it warm for 4 hours. aqueous solution.
Embodiment 3
[0024] Add 30 g of triazine ring residue, 900 g of water, and 1.5 g of copper sulfate to the reaction flask, start stirring, raise the temperature to 90° C. and keep it warm for 5 hours. aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com