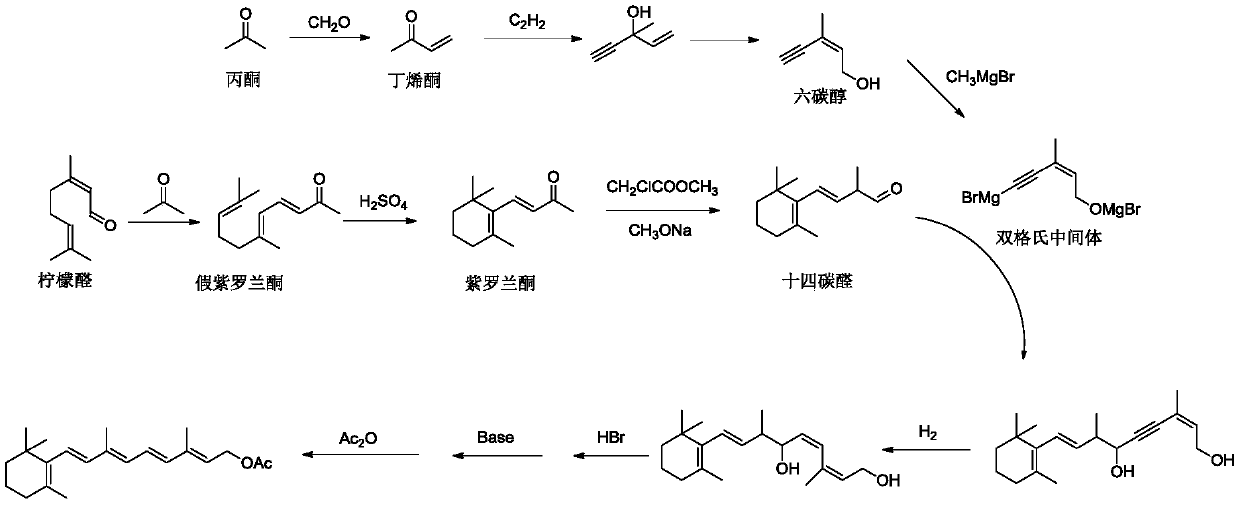
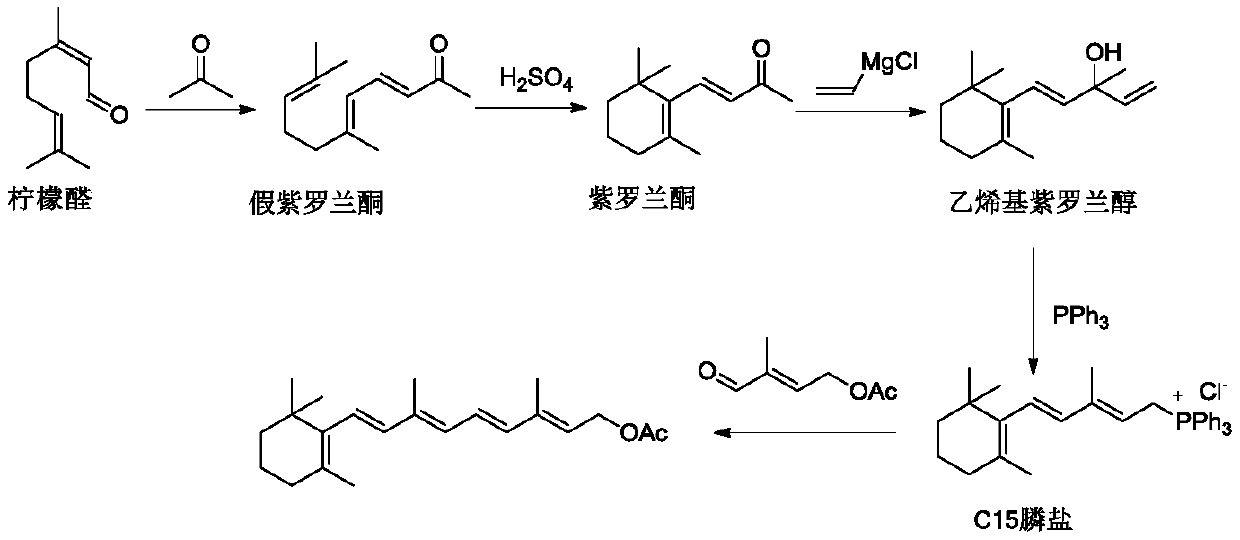
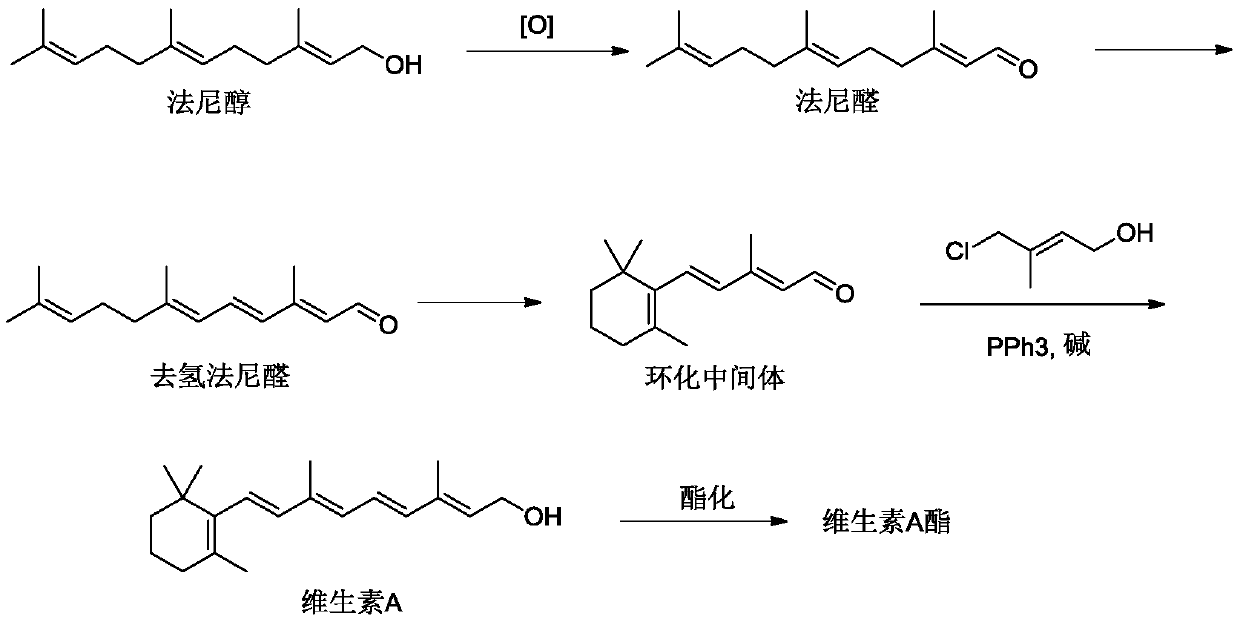
Method for preparing vitamin A and vitamin A ester
A vitamin and production method technology, which is applied in the fields of dehydrogenation preparation, carbon-based compound preparation, chemical instruments and methods, etc., can solve problems such as unfavorable industrial production and long steps, and achieves few reaction steps, mild reaction conditions, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Add 1170g of farnesol, 2L of DMF, 12g of TEMPO, and 12g of cuprous chloride into the reactor, stir, and raise the temperature to 50°C. Oxygen was continuously introduced, and the reaction was maintained at 50°C for 0.5h. After the reaction was completed, the solvent was removed and distillation under reduced pressure was carried out to obtain 1066 g of farnesal with a content of 98% and a yield of 95%. 1H NMR (300MHz, CDCl 3 ) characterization results are: δ=9.78(s,1H),5.75(m,1H),5.26(m,1H),5.16(m,1H),2.03(m,8H),1.86(s,3H),1.80 (s,6H),1.75(s,3H).
[0063] Add 1000g of farnesal, 1226g of DDQ, 4L of toluene into the reactor, and stir. Raise the temperature to 50°C and react for 0.5h. After the reaction was finished, the solvent was removed, and the dehydrofarnesal was distilled under reduced pressure to obtain 940 g of dehydrofarnesal with a content of 98% and a yield of 95%. 1 H NMR (300MHz, CDCl 3 ) characterization results are: δ=9.76(s,1H),6.53(m,2H),6.40(m,1H),...
Embodiment 2
[0068] Add 1170g of farnesol, 2L of DMF, 2.3g of 4-hydroxy-TEMPO, and 2.3g of cuprous chloride into the reactor, stir and keep at 30°C. Continue to feed air, and keep at 30°C for 2h. After the reaction was completed, the solvent was removed and distillation under reduced pressure was carried out to obtain 1090 g of farnesal with a content of 98% and a yield of 97%.
[0069] Add 1000g farnesal, 1162g chloranil, 4L tetrahydrofuran into the reactor, and stir. Keep at 30°C and react for 2h. After the reaction was completed, the solvent was removed, and the dehydrofarnesal was distilled under reduced pressure to obtain 931 g of dehydrofarnesal with a content of 98% and a yield of 94%.
[0070] Add 450g of phosphoric acid, 900g of dehydrofarnesal, and 1800g of toluene into the reactor, and stir. Raise the temperature to 50°C and react for 3h. After the reaction was completed, the temperature was lowered to room temperature, and the phases were separated. After removing the solv...
Embodiment 3
[0074] Add 1170g of farnesol, 2L of DMF, 1.2g of 4-methoxy-TEMPO, and 1.2g of cuprous chloride into the reactor, stir and keep at 40°C. Oxygen was continuously introduced, and the reaction was maintained at 40° C. for 1 h. After the reaction was completed, the solvent was removed and distillation under reduced pressure was carried out to obtain 1090 g of farnesal with a content of 98% and a yield of 97%.
[0075] Add 1000g farnesal, 1070g DDQ, 4L ethyl acetate into the reactor, and stir. Keep at 40°C and react for 1h. After the reaction was completed, the solvent was removed, and the dehydrofarnesal was distilled under reduced pressure to obtain 922g of dehydrofarnesal with a content of 98% and a yield of 93%.
[0076] Add 900g of trifluoromethanesulfonic acid, 900g of dehydrofarnesal, and 1800g of dichloromethane into the reactor, stir, and heat at 40°C for 3h. After the reaction was completed, the temperature was lowered to room temperature, and the phases were separated....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com