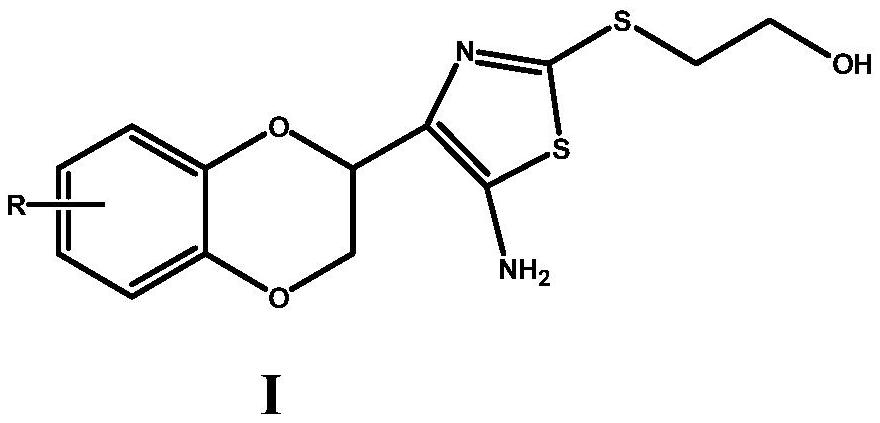
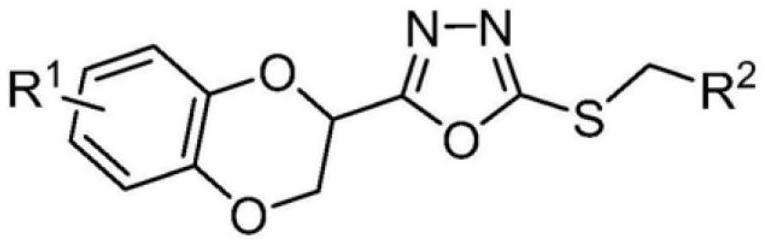
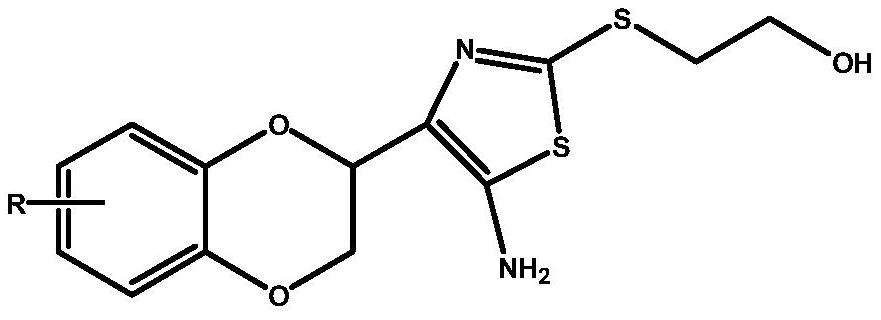
A kind of medicine for treating brain glioma and preparation method thereof
A technology of pharmacy and compound, applied in the field of pharmacy, can solve the problem of small dosage and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of compound I-a
[0033]
[0034] 1,4-benzodioxane-2-chloromethylamine (1.0mmol) and NaCN (1.05mmol) were dissolved in ethanol, and after reacting at room temperature for 5 hours, TLC detection showed that the reactant 1,4-benzo Dioxane-2-chloromethylamine disappeared, stop the reaction, add 20mL deionized water and 20mL dichloromethane, let stand to separate, collect the organic phase, distill off the solvent under reduced pressure, and proceed directly to the next reaction without purification .
[0035] Dissolve the product after cyano substitution in the previous step and 0.6 equivalent of sodium carbonate in acetonitrile solvent, slowly add carbon disulfide (1.1 equivalent) dropwise within half an hour, heat and reflux and stir for Cook-Heilbron reaction for 10 hours, TLC detects raw materials After disappearing, add an appropriate amount of deionized water, filter to remove insoluble matter, collect the filtrate, add dilute hydroc...
Embodiment 2
[0039] Embodiment 2: the preparation of compound I-b
[0040]
[0041] With 1,4-m-chlorobenzodioxane-2-chloromethylamine as the initial reactant, compound I-b was prepared by the same method as in Example 1, wherein in the (3) step, through flash column chromatography (two Chloromethane:methanol=10:3) to obtain 282 mg of white solid I-b (HPLC purity: 99.4%), with a total yield of 82%.
[0042] 1 H NMR (300MHz, CDCl 3 )δ8.57(s, 1H), δ8.32(d, 1H), 7.64(d, 1H), 7.46(t, 1H), 6.41(d, J 7.6Hz, 2H), 6.17(t, 1H) , 6.09(s, 2H), 5.81-5.96(m, 4H);
[0043] ESI MS m / z 345.0[M+Na] + .
Embodiment 3
[0044] Embodiment 3: the preparation of compound I-c
[0045]
[0046] Using 1,4-m-methoxybenzodioxane-2-chloromethylamine as the initial reactant, the same method as in Example 1 was used to prepare compound I-b, wherein in step (3) flash column chromatography (Dichloromethane:methanol=5:1) to obtain 303 mg of white solid I-c (HPLC purity: 99.8%), with a total yield of 89%.
[0047] 1 H NMR (300MHz, CDCl 3 )δ8.36(s, 1H), δ8.12(d, 1H), 7.57(d, 1H), 7.28(t, 1H), 6.37(d, J 7.1Hz, 2H), 6.29(t, 1H) , 6.02(s, 2H), 5.77-5.97(m, 4H), 4.79(s, 3H);
[0048] ESI MS m / z 341.0[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com