The use of the compound
A technology of compounds and uses, applied in the field of pharmaceutical use of compounds, to achieve the effects of inhibiting migration, good anti-angiogenic activity, and promoting cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
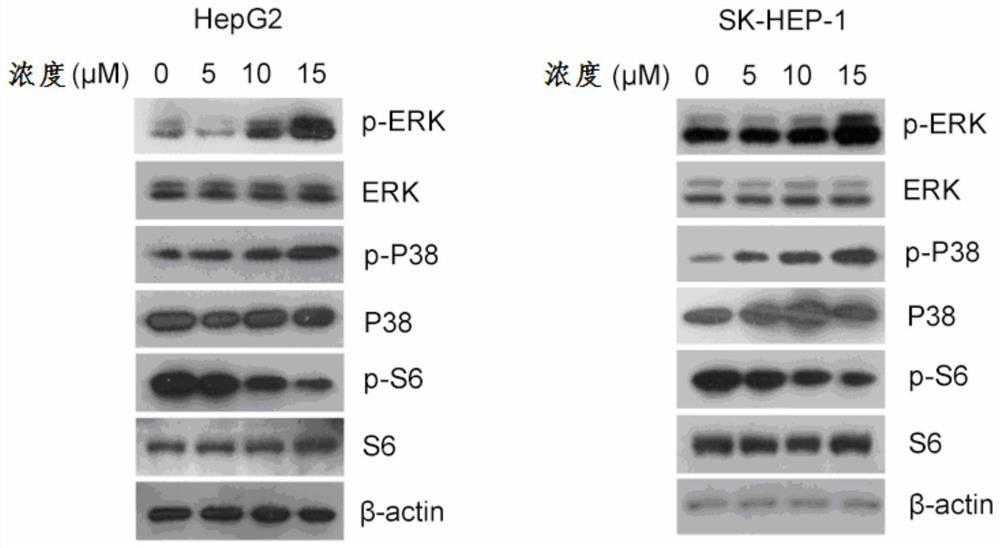
[0039] Embodiment 1-cell proliferation experiment
[0040] experimental method:
[0041] HepG2 and SKHEP-1 cells in the logarithmic growth phase were taken, and cell suspensions were prepared respectively, and the number of cells was adjusted to 3.5×10 4 A / mL~4.0×10 4cells / mL, added to 96-well plate, 100 μl per well. The cells were cultured for 24 hours to make them completely adhere to the wall, and the mother solution of the compound represented by formula (I) was diluted with complete medium into working solutions of different concentrations, and 5 replicate wells were set for each concentration. After 24 hours, 48 hours, and 72 hours after the addition of the drug, the original medium in the well plate was precipitated, and the 5mg / ml MTT reagent was prepared into a 10% concentration with the basal medium and added to the 96-well plate. The plate was incubated in a floating chamber at 37°C for 4 hours, then taken out, and 150 μl of DMSO was added to each well, disso...
Embodiment 2
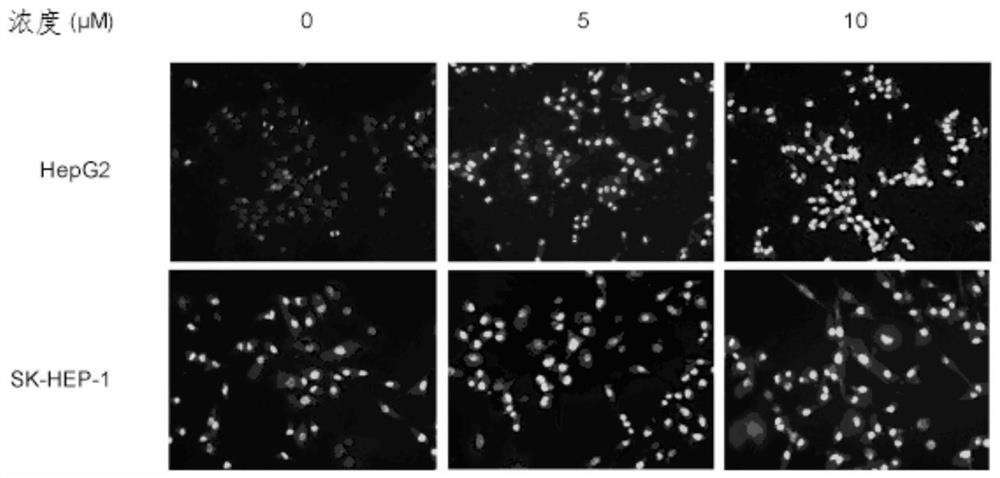
[0046] Embodiment 2-apoptosis experiment
[0047] experimental method:
[0048] (1) Hoechst staining method: Take HepG2 and SKHEP-1 cells in the logarithmic growth phase, pipette them into a single cell suspension with complete medium, inoculate them in a 12-well plate, and culture them in an incubator for 24 hours to make them adhere to the wall Add different concentrations of compounds represented by formula (I) and incubate for 48 hours, wash twice with PBS, fix with 4% paraformaldehyde for 10 minutes, and stain with Hoechst 33258 dye solution with a final concentration of 1 μg / ml in the dark for 30 minutes, place Under an inverted fluorescent microscope, observe and take pictures. Experiments were repeated at least three times independently.
[0049] (2) Flow cytometry assay: HepG2 and SKHEP-1 cells were inoculated in 6-well plates at a concentration of 1×105 / mL, and after overnight attachment, the final concentrations of 0, 5, 10, and 15 μM were added to the formula (...
Embodiment 3
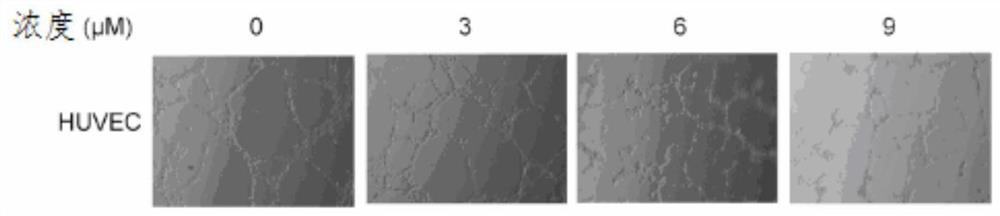
[0055] Embodiment 3-cell scratch experiment
[0056] experimental method:
[0057] HepG2 and SKHEP-1 cells were seeded in 12-well plates, and when the cells were in a subconfluent state, they were starved for 12 hours with serum-free DMEM high-sugar medium. Use a 10 μl white pipette tip to scratch vertically and gently with even force. 1×PBS was used to wash the cell debris floating due to scratching, at least 3 times. Take pictures as a blank control before dosing. Dilute the mother solution of the compound represented by formula (I) into working solutions of 0, 2, 4 and 8 μM with serum-free basal medium, add drugs to stimulate the cells respectively, observe and take pictures under a phase contrast microscope 12 hours and 24 hours after scratching, and evaluate The size of the cell migration ability.
[0058] Experimental results:
[0059] The cell scratch test was used to evaluate the migration ability of cells in vitro. The effects of different concentrations of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com