A kind of anti-tumor small molecular polypeptide and its application
A small molecule polypeptide, anti-tumor technology, applied in the field of biotechnology and medicine, can solve problems such as large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
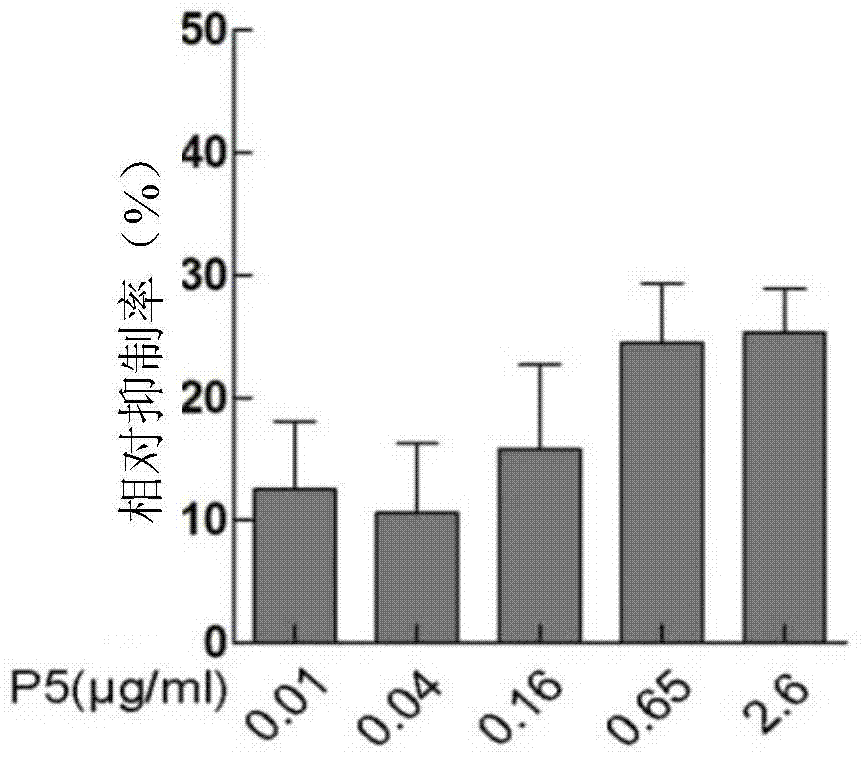
[0022] Example 1 Design and Synthesis of Small Molecular Peptides and Detection of Antitumor Effects
[0023] (1) Design of small molecule peptides
[0024] The present invention adopts the method of bioinformatics, according to the FGF2-FGFR2 protein binding mode, and based on the FGF2-FGFR2 interaction site, designs small molecular polypeptides, and the key sites on FGF2 that interact with FGFR2 are mainly: Tyr-24, Phe -31, Gln-56, Vla-57, Glu-58, Tyr-73, Val-88, Phe-93, Asn-102, Asn-104, Leu-140, Met-142; key to the interaction with FGF2 on FGFR2 The sites are: Leu-166, Ala-168, Pro-170, Val-249, Arg-251, Asp-283, Val-317, Asn-318, Asp-321. Accerly's protein simulation software Discovery Studio2.5 / 3.5 was used to analyze the binding of FGF2 and its specific binding receptor FGFR2IIIc, and each polypeptide contained 1 to 3 key amino acids on FGF2 that interact with FGFR2, and It is required that the length of the polypeptide just falls into the active pocket of the FGFR2 p...
Embodiment 2
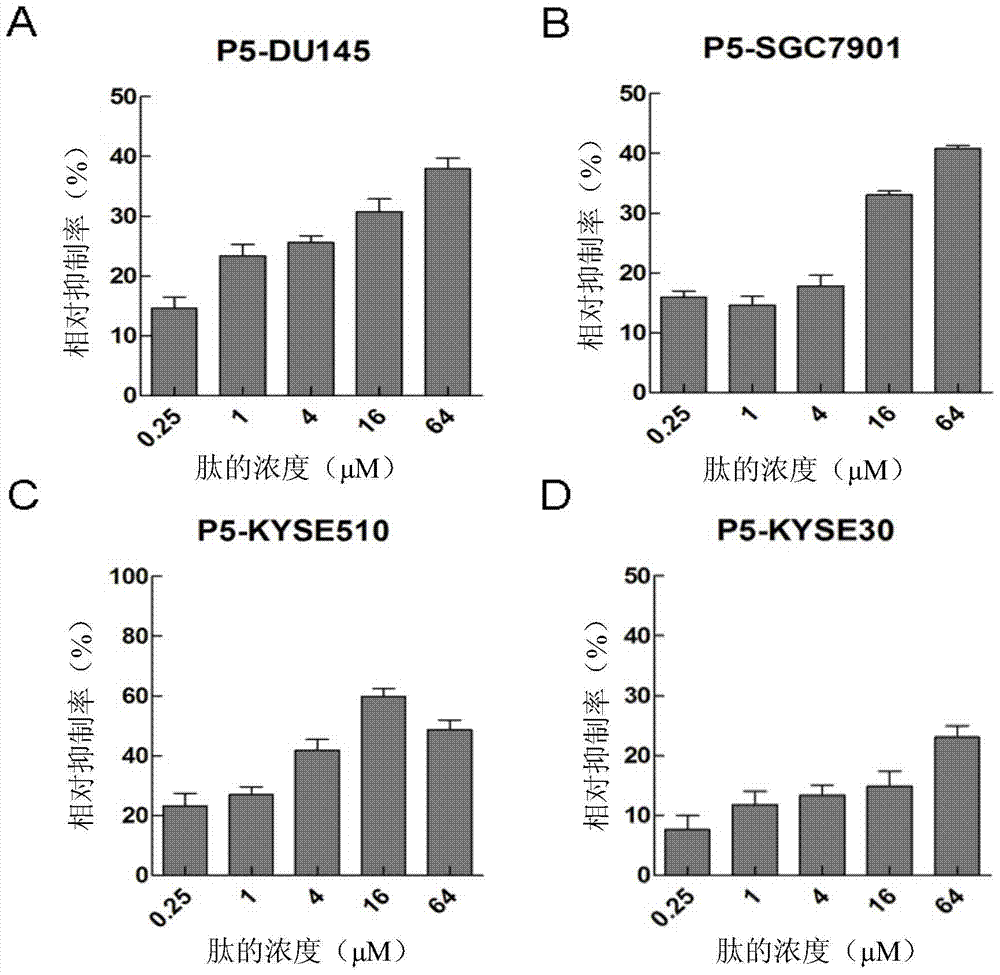
[0040] Example 2: Detection of broad-spectrum anti-tumor properties of small molecule polypeptide P5
[0041] (1) Select the test cell line
[0042]The candidate cell lines are DU145 (prostate cancer cell line), SGC7901 (gastric adenocarcinoma cell line), KYSE30 (human esophageal squamous cell line), and KYSE510 (human esophageal line cell line), all of which were purchased from Shanghai Cell Bank.
[0043] (2) Test method
[0044] The test method is the same as in Example 1, and the logarithmic phase cell lines are DU145, SGC7901, KYSE30, and KYSE510.
[0045] (3) Test results
[0046] Such as figure 2 As shown, P5 has a certain level of inhibition on the proliferation of the above four cell lines, and all of them are concentration-gradient dependent. Except for KYSE510 cells, the inhibition rate of other cells has not reached the maximum efficiency. This experiment shows that the inhibitory rate of P5 to the four cell lines is more obvious for DU145, SGC7901, and KYSE51...
Embodiment 3
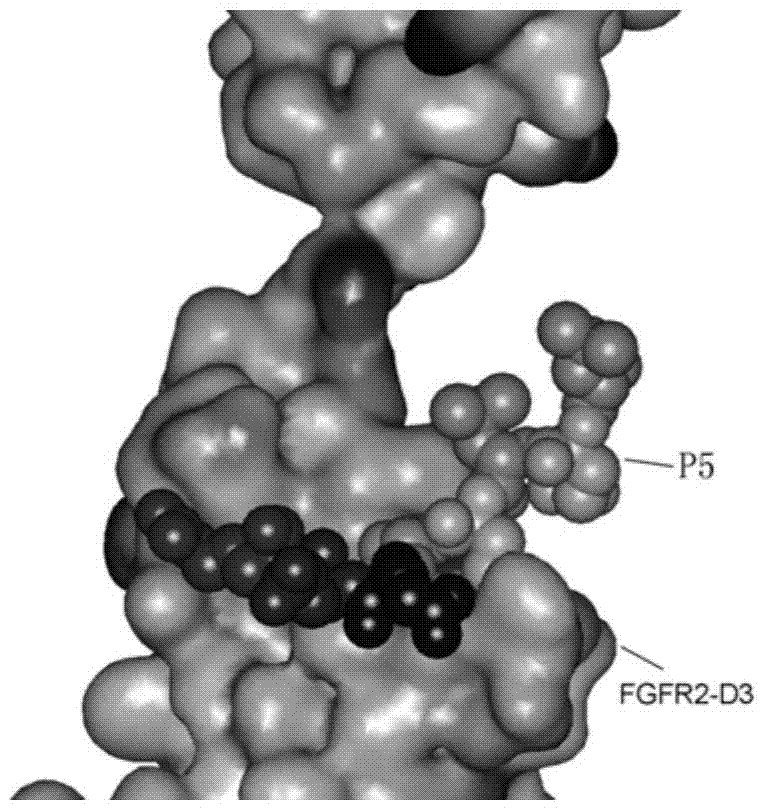
[0047] Example 3: Analysis of the binding site of P5 peptide on FGFR2 protein
[0048] (1) Select the test cell line
[0049] The P5 peptide has the greatest effect on the growth and proliferation of the DU145 cell line in inhibiting FGFR2. In the subsequent study of the function and molecular mechanism of the P5 peptide in inhibiting cell proliferation, the target cell line is DU145.
[0050] (2) Test methods and results
[0051] ①Discovery Studio 2.5 software opens the FGF2-FGFR2 protein structure (PDB number: 1EV2), finds the homologous sequence of P5 (53-60 amino acids) on the FGF2 protein, selects the FGFR2 protein, adds a surface layer on the surface of the FGFR2 protein, and selects P5 homologous sequence, change the P5 homologous sequence into CPK mode, such as image 3 structure shown. From the results, it can be seen that amino acids such as (Gln-56, Ala-57, Glu-58, Glu-59) in the homologous sequence of P5 peptide just fall in the D3 domain of FGFR2 protein and ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com