Method for diagnosis of bile duct cancer using methionyl-trna synthetase in bile duct cell
A technology of methionyl and bile duct cells, applied in the field of diagnosis of cholangiocarcinoma, can solve the problems of no markers and pathological diagnosis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
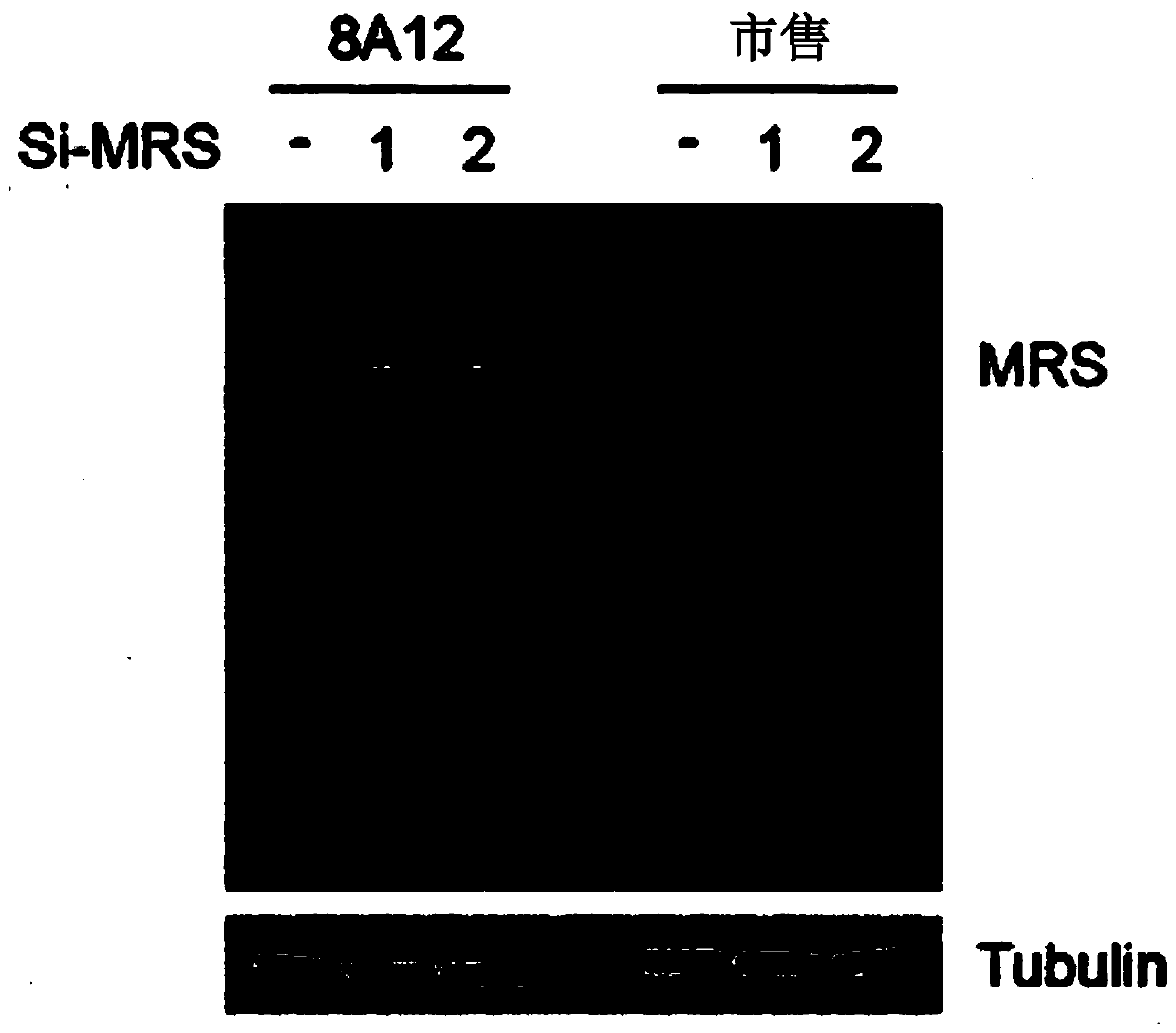
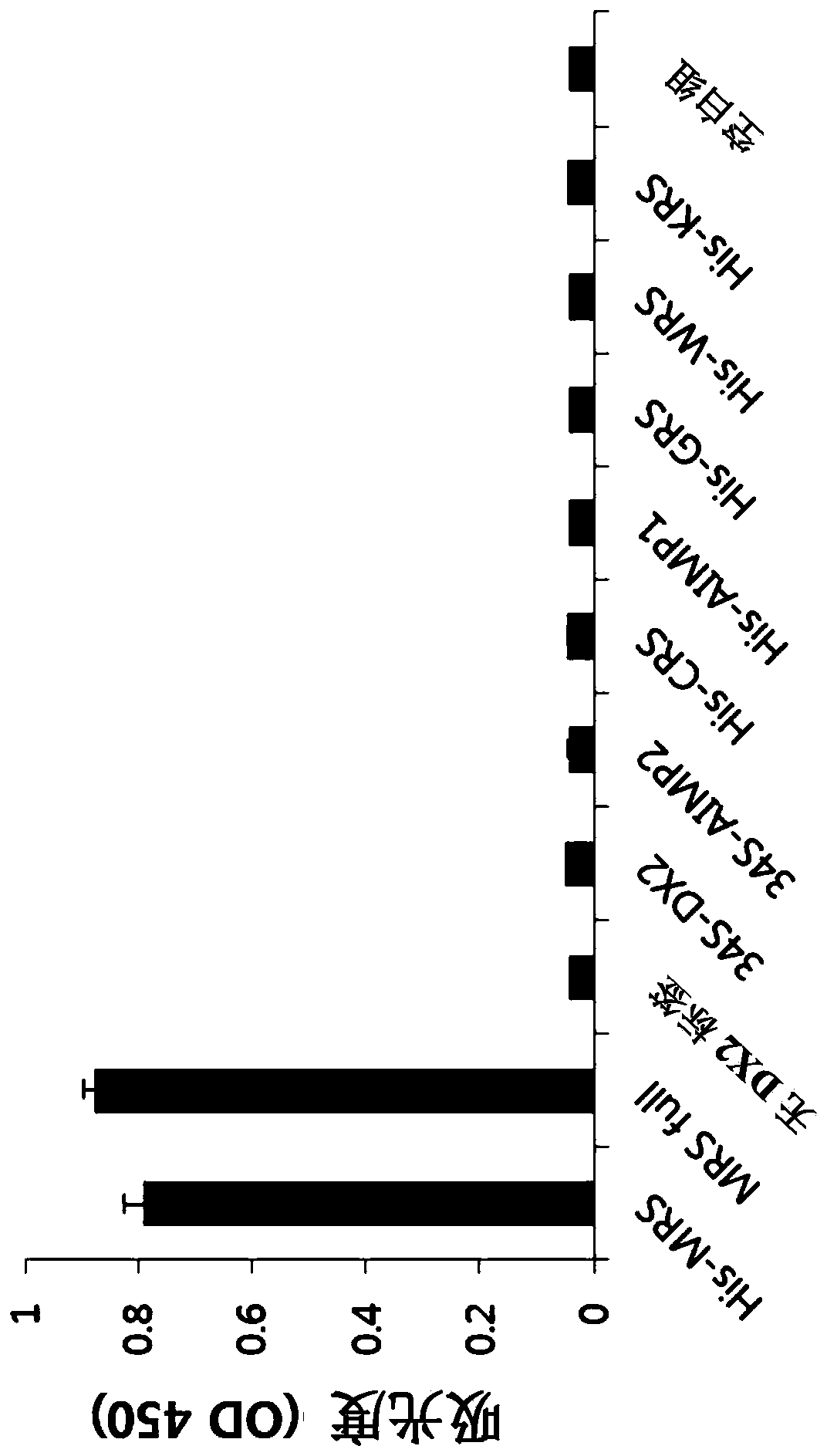
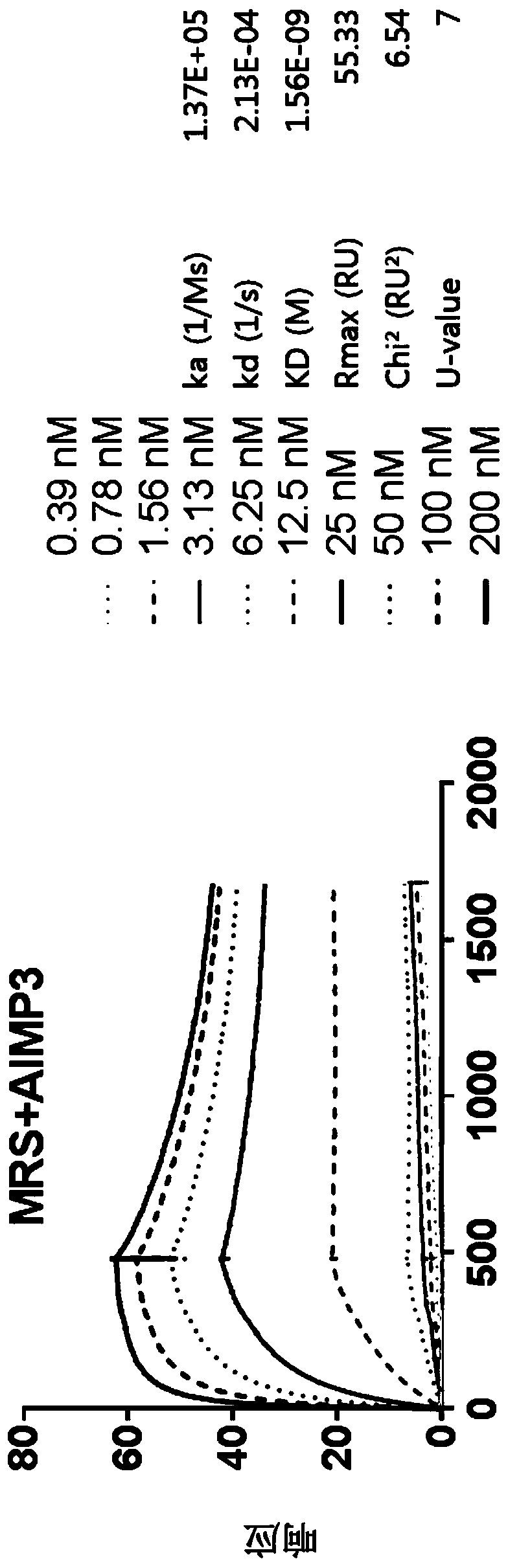
[0136] Example 1: The present invention is used for the construction of the antibody of cholangiocarcinoma inspection (obtaining the antibody with high specificity to MRS)
[0137] It is known that in vivo, methionyl-tRNA synthetase (MRS) exists in a state bound to aminoacyl-tRNA synthetase complex-interacting multifunctional protein 3 (AIMP3), and this binding is disrupted by UV irradiation or the like. Thus, for substantially accurate detection of MRS, only the detection of MRS specifically is required, even in the case of MRS binding to AIMP3. However, the protein structures between current AIMP types and ARS types have many similarities, so commercially available antibodies have the problem of showing cross-activity with different AIMP and ARS types. For the diagnostic accuracy of the cholangiocarcinoma examination method of the present invention, the present inventors prepared a highly sensitive MRS antibody that has no cross activity with other proteins as follows.
[0...
Embodiment 2
[0179] Example 2: Establishment of a specific MRS expression detection method (staining method) for cholangiocarcinoma cells in cell diagnosis and confirmation of its effect
[0180] 1) Obtain specimens according to commonly used brush cytology (Osnes M, Serck-Hanssen A, Myren J. Scand J Gastroenterol. 1975; 10(8):829-31). Specifically, bile duct brushing was performed using a GRBH-230-3-3.5 brush (Wilson-Cook Medical, Inc., Winston-Salem, NC). Allow the brush to move back and forth over the lesion five to eight times. Afterwards, the brushes were washed with Roswel Park Memorial Institute (RPMI) 1640 medium (GibcoBRL, Rockville, MA, USA) and immediately moved to the cytology laboratory for liquid-based cytology (Thinprep). Shake the brush in ThinPrep fixative solution (PreservvCyt solution) to release cholangiocytes. The sample thus obtained (cholangiocytes) was spread on a ThinPrep glass slide by a conventional method using ThinPrep (Hologic Inc) to prepare a cell sample. ...
Embodiment 3
[0203] Example 3: In biopsy, the establishment of a specific MRS expression detection method for cholangiocarcinoma cells and the confirmation of its effect
[0204] Immunohistochemistry (IHC) for determining the extent of MRS expression in cholangiocarcinoma tissue and normal bile duct tissue (including benign bile duct stricture cells but not cancer) was developed as follows. Specifically, typically 55 unknown bile duct biopsy tissue samples were paraffin-embedded and sectioned. Afterwards, the final samples are obtained by processing in the following order:
[0205] ① Soak sliced tissue in xylene for 24 hours → treat with 100% alcohol twice for 2 minutes → treat with 95% alcohol once for 2 minutes → treat with 90% alcohol twice for 2 minutes → treat with 70% alcohol once for 2 minutes → wash with DW two or three times;
[0206] ② Antigen recovery: dilute commercially available citrate buffer (DW 9: citrate buffer 1), preheat for 2 minutes, put slides in it, and heat for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com