Cetylpyridinium chloride buccal tablet and preparation technology thereof
A cetylpyridinium chloride, preparation process technology, applied in the direction of medical preparations containing active ingredients, medical preparations without active ingredients, pill delivery, etc., can solve the problems of complicated preparation process, and achieve easy preparation process and high product quality Stable and reliable, the effect of improving medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
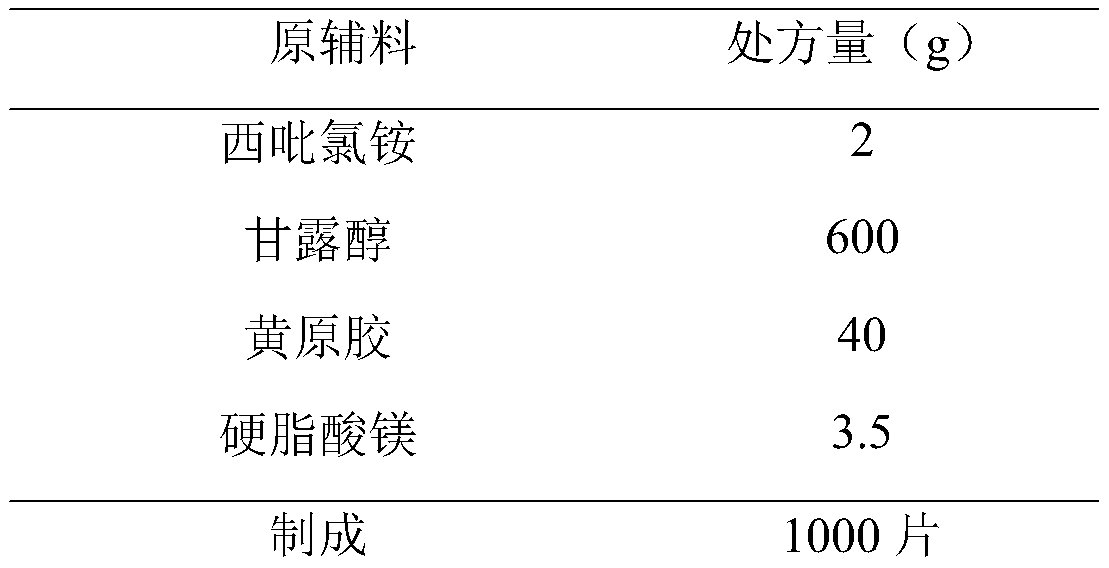
[0027]
[0028] Preparation Process:
[0029] 1) Pretreatment of raw and auxiliary materials: Cetylpyridinium chloride, xanthan gum, and mannitol are passed through a 80-mesh sieve, and magnesium stearate is passed through a 100-mesh sieve.
[0030] 2) Weighing: Take cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate according to the prescription.
[0031] 3) Total blending: put the prepared cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate into the three-dimensional mixer, and mix for 45 minutes.
[0032] 4) tableting and packaging.
Embodiment 2
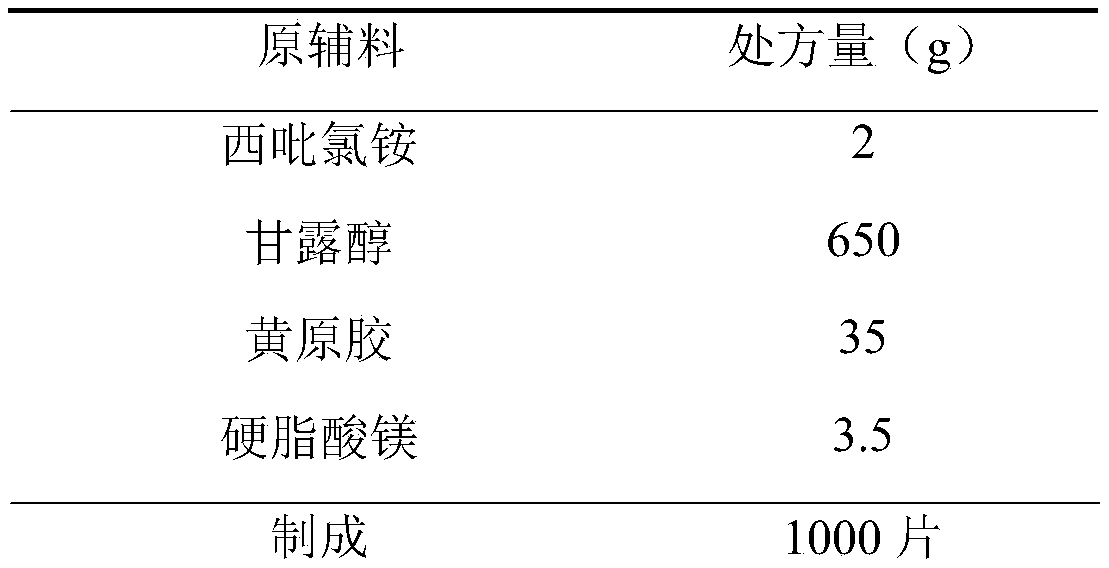
[0034]
[0035] Preparation Process:
[0036] 1) Pretreatment of raw and auxiliary materials: Cetylpyridinium chloride, xanthan gum, and mannitol are passed through a 80-mesh sieve, and magnesium stearate is passed through a 100-mesh sieve.
[0037] 2) Weighing: Take cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate according to the prescription.
[0038] 3) Total blending: put the prepared cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate into the three-dimensional mixer, and mix for 45 minutes.
[0039] 4) tableting and packaging.
Embodiment 3
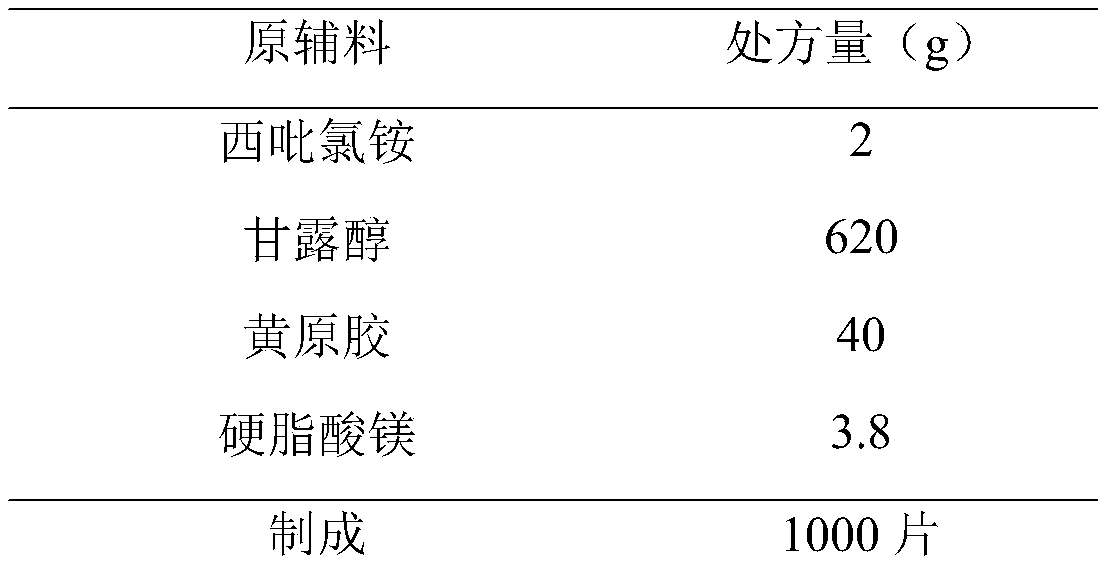
[0041]
[0042] Preparation Process:
[0043] 1) Pretreatment of raw and auxiliary materials: Cetylpyridinium chloride, xanthan gum, and mannitol are passed through a 80-mesh sieve, and magnesium stearate is passed through a 100-mesh sieve.
[0044] 2) Weighing: Take cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate according to the prescription.
[0045] 3) Total blending: put the prepared cetylpyridinium chloride, xanthan gum, mannitol and magnesium stearate into the three-dimensional mixer, and mix for 45 minutes.
[0046] 4) tableting and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com