Nucleotide molecule for in-vitro transcription of mRNA, presenting cell and application
An in vitro transcription and nucleotide technology, applied in the field of abnormal protein expression of disease antigens and applications, can solve the problems of not meeting the needs of disease treatment, unable to produce stable antigen presentation ability, poor mRNA stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
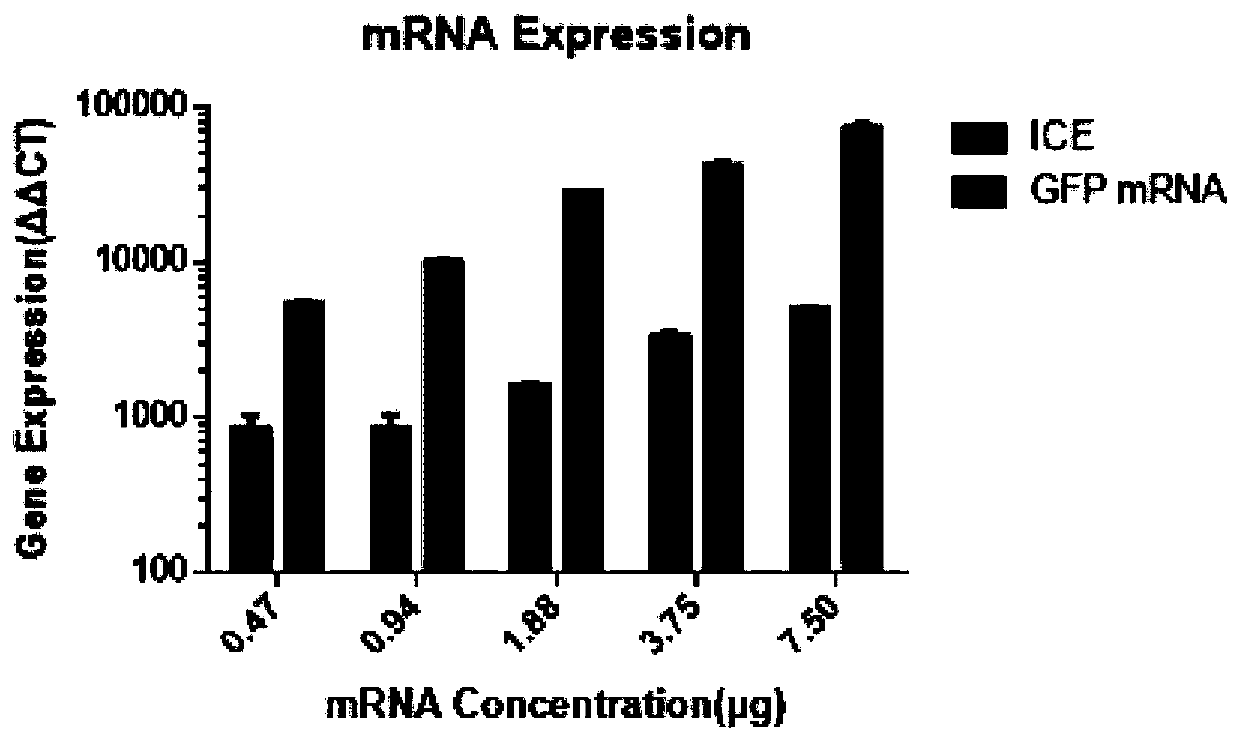
[0075] The acquisition of the nucleotide molecule of embodiment 1 in vitro transcription mRNA
[0076] 1. Codon optimization
[0077] Use the codon optimization software and website to optimize the codons of the N-terminal signal peptide of wild-type human MHC class I, the coding region of the human class I MHC transport signal sequence, and the coding region of the CEF epitope to obtain the N-terminal of MHC class I. Terminal signal peptide, nucleotide sequence of human MHC class I transit signal sequence, CEF epitope.
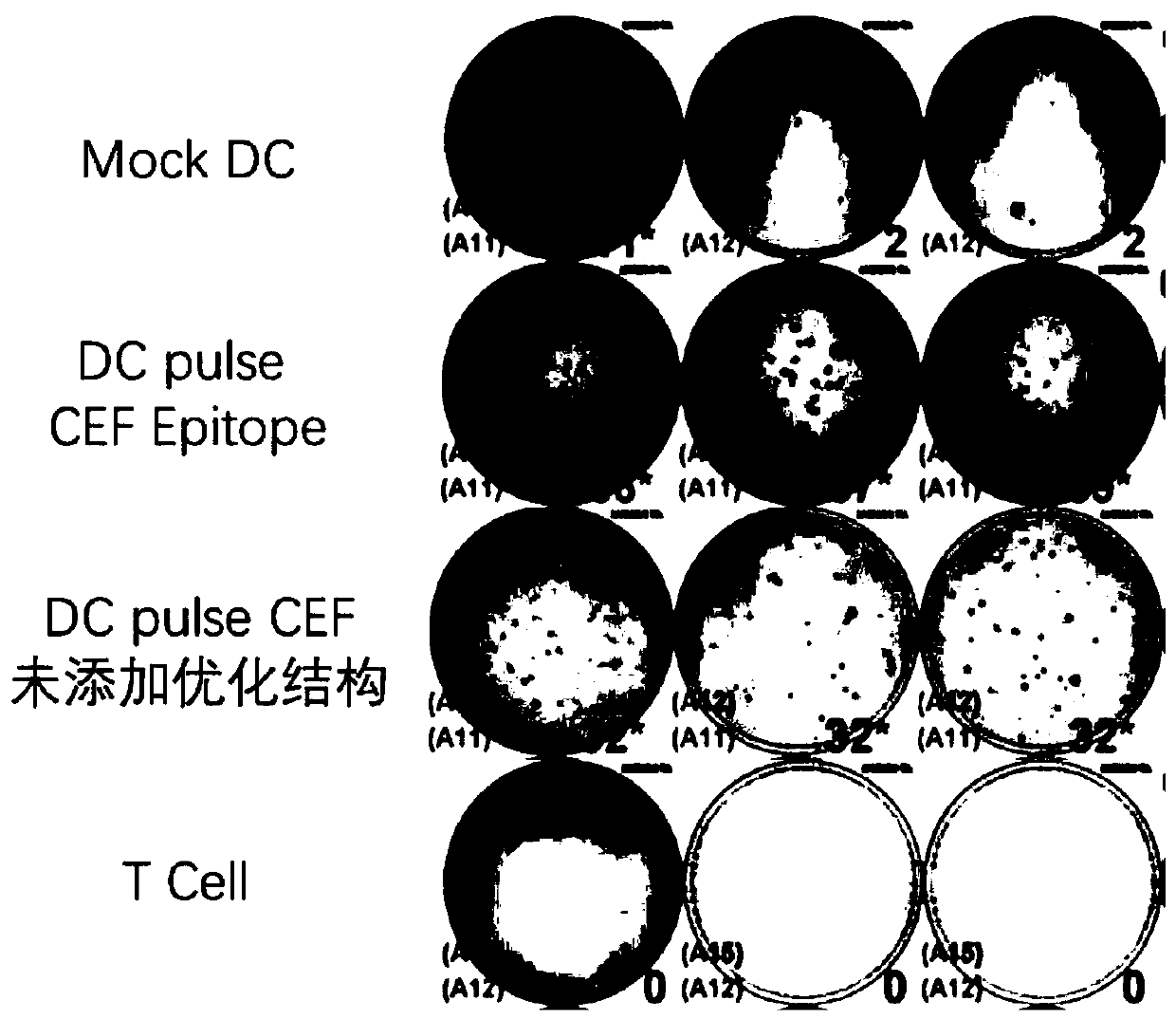
[0078]It should be noted that, in one embodiment of the present invention, the CEF epitope is only an exemplary epitope, and those skilled in the art can use other epitopes with tandem lymphocytes as nucleotide analysis tandem epitope elements to replace CEF epitope in this example.
[0079] The CEF epitopes used in the present invention are positive epitopes of Cytomegalovirus, Epstein-Barr Virus and InfluenzaVirus Control Peptide, each epitope is 8-12 ami...
Embodiment 2
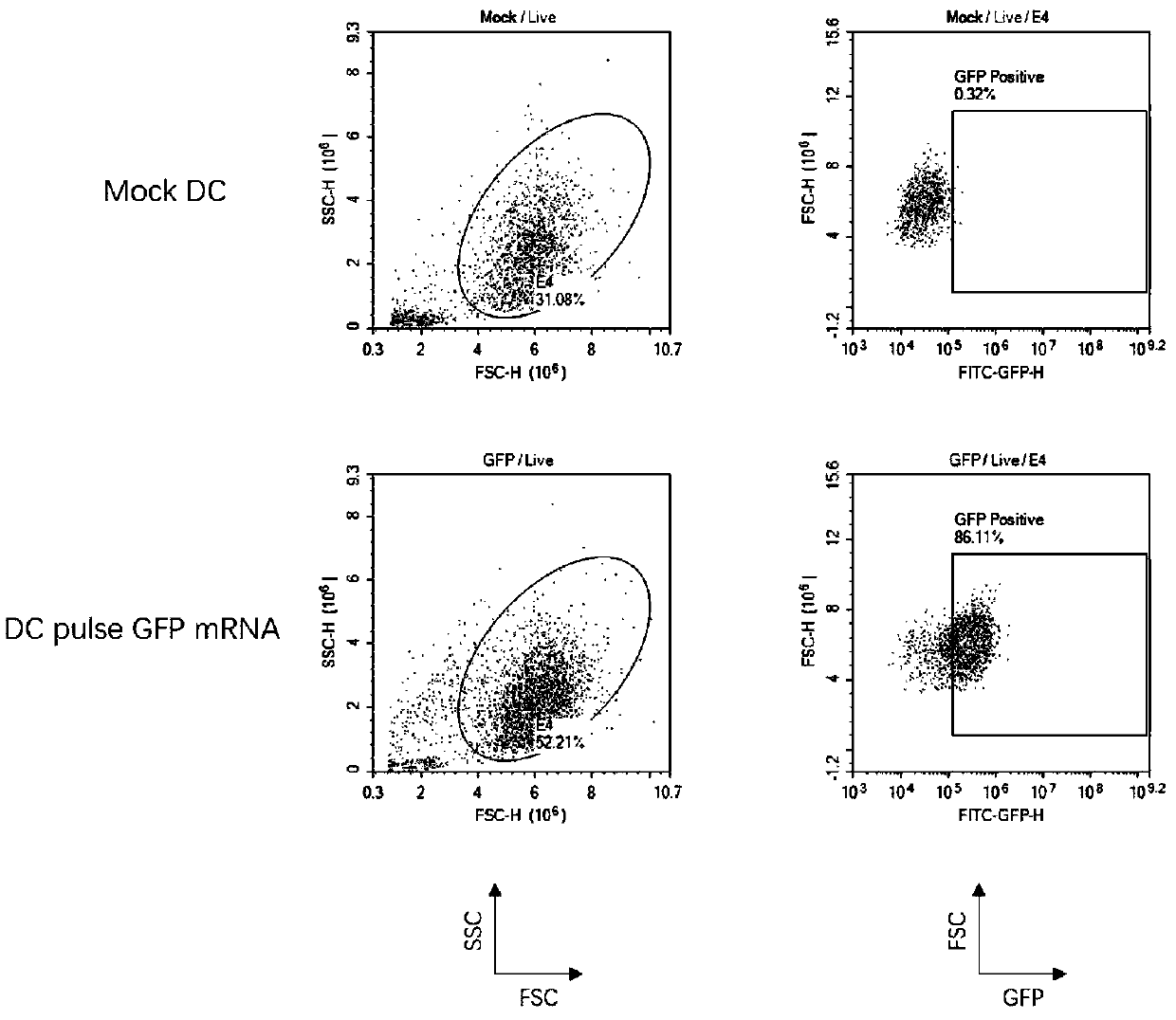
[0104] Example 2 Antigen Presenting Cells
[0105] 1. Induction and culture of DC in vitro
[0106] 1.1 Isolation of high-purity monocytes
[0107] In order to obtain higher purity mononuclear cells, the mononuclear cells in the peripheral blood of healthy volunteers were firstly separated and purified by a mononuclear cell collection machine, and then the peripheral blood mononuclear cells were separated by lymphocyte separation medium in an ultra-clean bench. Select the kit to purify monocytes to obtain high-purity monocytes.
[0108] 1.2 Culture of DC
[0109] Adding monocytes to ImmunoCult TM Differentiation medium (ImmunoCult TM Add ImmunoCult to the medium TM DC Differentiation Medium), placed at 37°C, 5% CO 2 Cultured in the incubator for 6 days, half of the medium was changed every 3 days.
[0110] Perform maturation culture of DC on day 6 and replace with ImmunoCult TM In maturation medium (ImmunoCult TM Add ImmunoCult to the medium TM DC Maturation Medium),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com