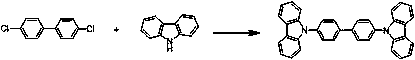Preparation method of 4,4'-dicarbazolyl biphenyl
A technology of carbazolyl biphenyl and dichlorobiphenyl, applied in organic chemistry and other directions, can solve the problem of low nucleophilic ability of N atom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under an inert gas atmosphere, add a stirring bar to a dry 50 mL Schlenk bottle, add carbazole (0.368 g, 2.2 mmol) and xylene (3 mL), cool to 5 ° C, and dropwise add methylmagnesium chloride (3.0 M in THF, 2.3mmol, 0.77 mL) (about 1.5 min), stirred and reacted for 15 min after the addition, and transferred to the glove box for later use.
[0026] In the glove box, add 4,4'-dichlorobiphenyl (0.223 g, 1.0 mmol), the complex [2,6-bis(2,4,6-triisopropylphenyl ) phenyl-dicyclohexylphosphine] (allyl- ) palladium(II) chloride (0.86 mg, 0.001 mmol), 2,6-bis(2,4,6-triisopropylphenyl)phenyl-dicyclohexylphosphine (0.68 mg, 0.001 mmol) and 0.13 mL of dodecane (as an internal standard for GC analysis) was dissolved in 1 mL of xylene. At room temperature, the reaction solution in the Schlenk bottle was transferred to a pressure-resistant tube, sealed, and reacted in an oil bath at 145 °C for 3 h. The reaction solution was filtered through silica gel and diatomaceous earth, the fi...
Embodiment 2
[0030]Under an inert gas atmosphere, add a stirring bar to a dry 50 mL Schlenk bottle, add carbazole (0.368 g, 2.2 mmol) and xylene (3 mL), cool to 5 ° C, and dropwise add methylmagnesium chloride (3.0 M in THF, 2.3mmol, 0.77 mL) (about 1.5 min), stirred and reacted for 15 min after the addition, and transferred to the glove box for later use.
[0031] In the glove box, add Pd(dba) to a pressure tube 2 (1.15 mg, 0.002 mmol), 2,6-bis(2,4,6-triisopropylphenyl)phenyl-dicyclohexylphosphine (2.72 mg, 0.004 mmol) were dissolved in 1 mL xylene, and then at 100 o After heating at C for 2 minutes, cool to room temperature, add 4,4’-dichlorobiphenyl (0.223 g, 1.0 mmol) and 0.13 mL dodecane (as internal standard for GC analysis). At room temperature, the reaction solution in the Schlenk bottle was transferred to a pressure-resistant tube, sealed, and reacted in an oil bath at 145 °C for 12 h. The reaction solution was filtered through silica gel and diatomaceous earth, the filtrate wa...
Embodiment 3
[0033] Under an inert gas atmosphere, add a stirring bar to a dry 50 mL Schlenk bottle, add carbazole (0.368 g, 2.2 mmol) and xylene (3 mL), cool to 5 ° C, and dropwise add methylmagnesium chloride (3.0 M in THF, 2.3mmol, 0.77 mL) (about 1.5 min), stirred and reacted for 15 min after the addition, and transferred to the glove box for later use.
[0034] In the glove box, add Pd(OAc) to a pressure tube 2 (0.67 mg, 0.003 mmol), 2,6-bis(2,4,6-triisopropylphenyl)phenyl-dicyclohexylphosphine (4.1 mg, 0.006 mmol) and water (0.27 uL, 1.5 mol% , 5.0eq) was dissolved in 1 mL xylene, then in 100 o Heat at C for 3 minutes, cool to room temperature, add 4,4’-dichlorobiphenyl (0.223 g, 1.0 mmol) and 0.13 mL dodecane (as internal standard for GC analysis). At room temperature, the reaction solution in the Schlenk bottle was transferred to a pressure-resistant tube, sealed, and reacted in an oil bath at 145 °C for 12 h. The reaction solution was filtered through silica gel and diatomaceou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com