A group of markers for predicting the curative effect of nasopharyngeal carcinoma induced chemotherapy, and applications thereof
A marker and nasopharyngeal carcinoma technology, applied in the field of biomarkers, can solve the problems of limited benefit, achieve the effect of accurately inducing chemotherapy efficacy and guiding clinical medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
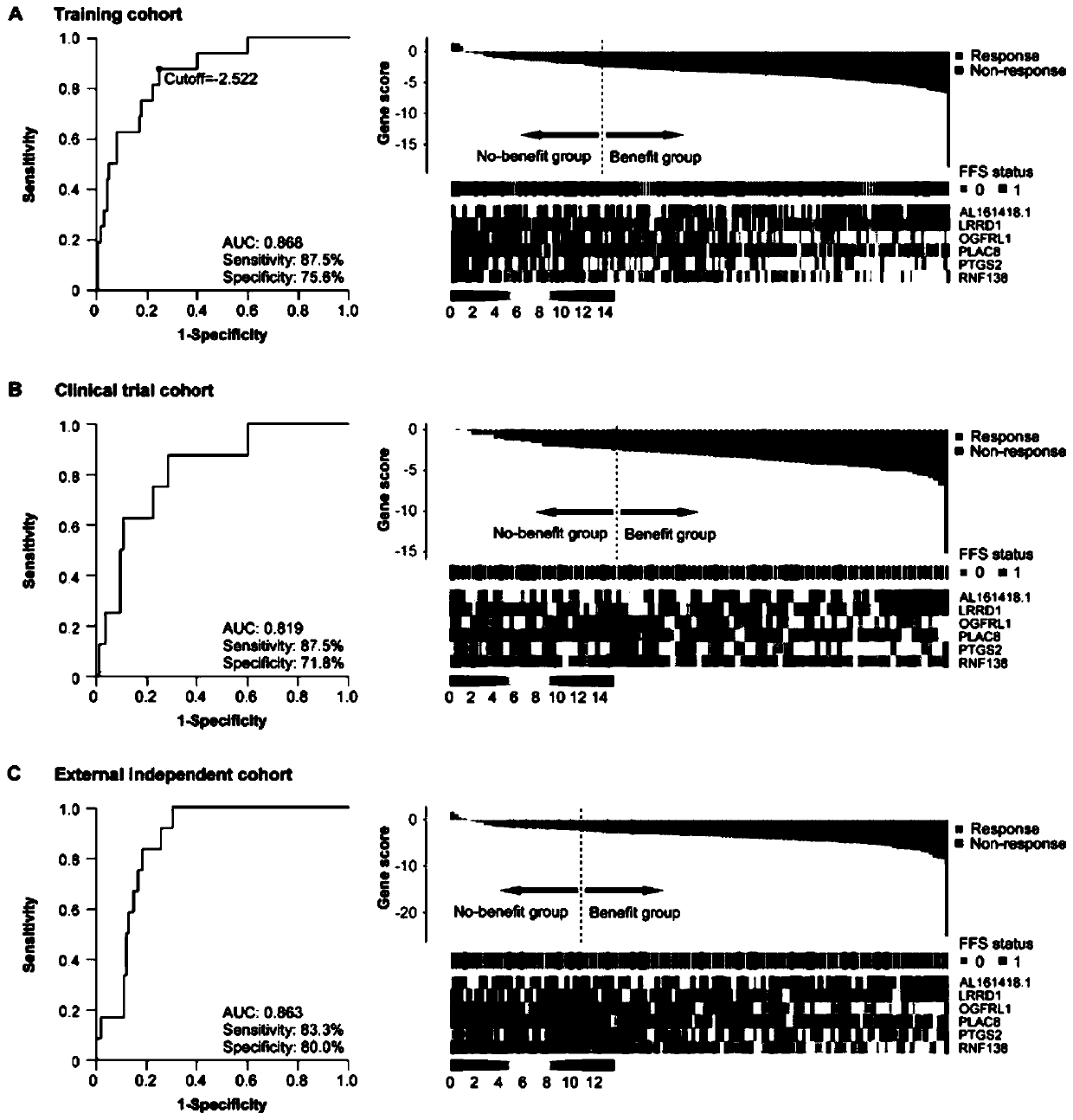
[0030] Example 1 Discovery of Molecular Targets for Nasopharyngeal Carcinoma Induction Chemotherapy
[0031] The inventor used the NanoString nCounter technology platform to detect these 43 differentially expressed genes and 5 internal reference genes in 342 samples of the training group (from Sun Yat-sen University Cancer Prevention and Control Center), and screened out 6 genes most related to the efficacy of induction chemotherapy to construct a model. The inventor named this group of 6-gene markers as 6-gene signature, and its calculation formula is:
[0032] 6-Gene signature score = -26.01361+(0.36816×AL161418.1 expression)-(0.64682×LRRD1 expression)+(0.70213×OGFRL1 expression)+(0.07571×PLAC8 expression)+(0.24862×PTGS2 expression )+(1.54186×RNF138 expression level).
[0033] The ROC curve was used to generate a threshold value of -2.522, thus 243 patients (71.1%) were divided into the benefit group, and 99 patients (28.9%) were divided into the non-benefit group; AUC rea...
Embodiment 2
[0034] Example 2 Further verification of molecular targets for nasopharyngeal carcinoma induction chemotherapy efficacy
[0035] The inventors then verified this set of molecular labels in samples from this hospital and a set of samples from other hospitals.
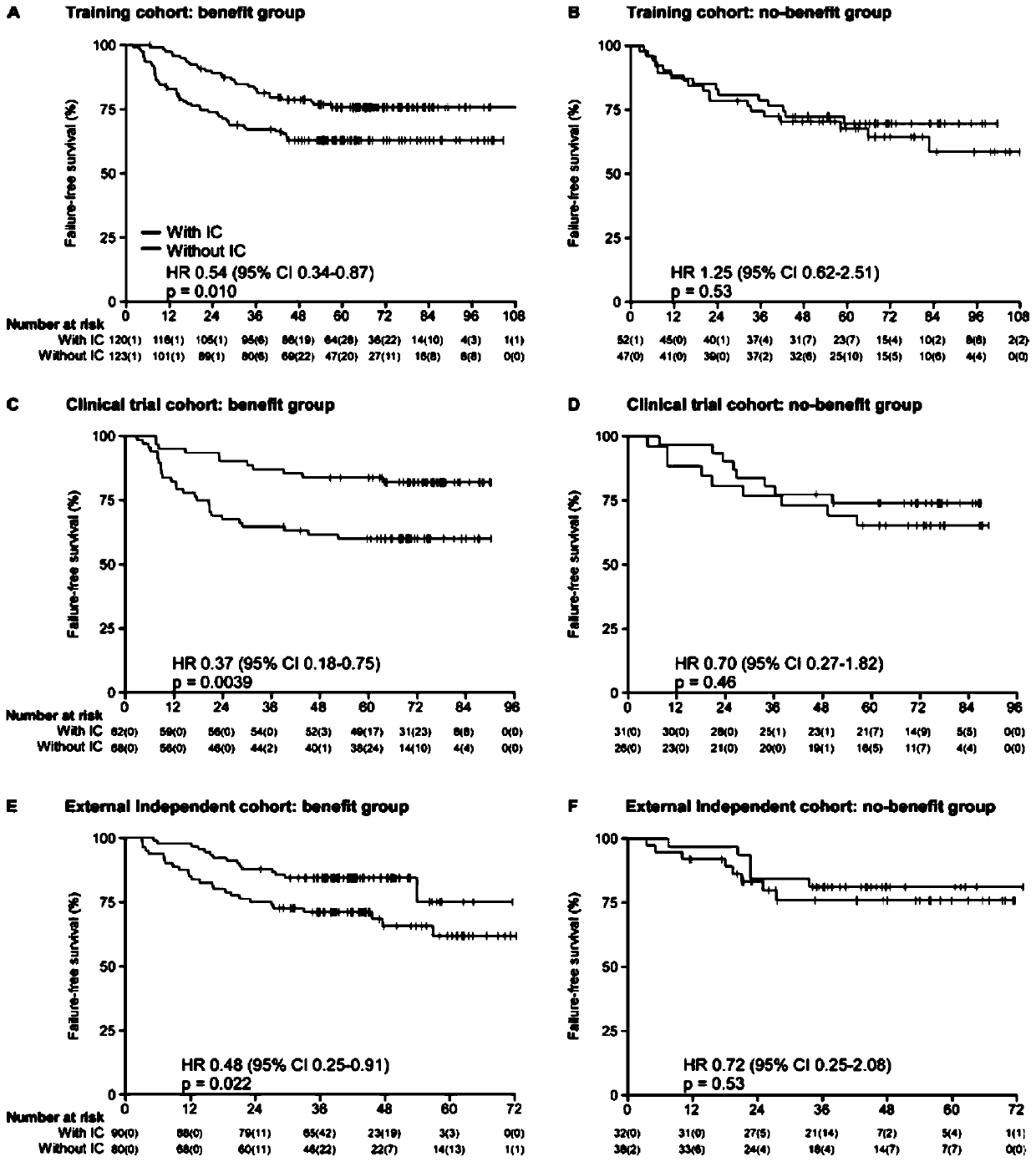
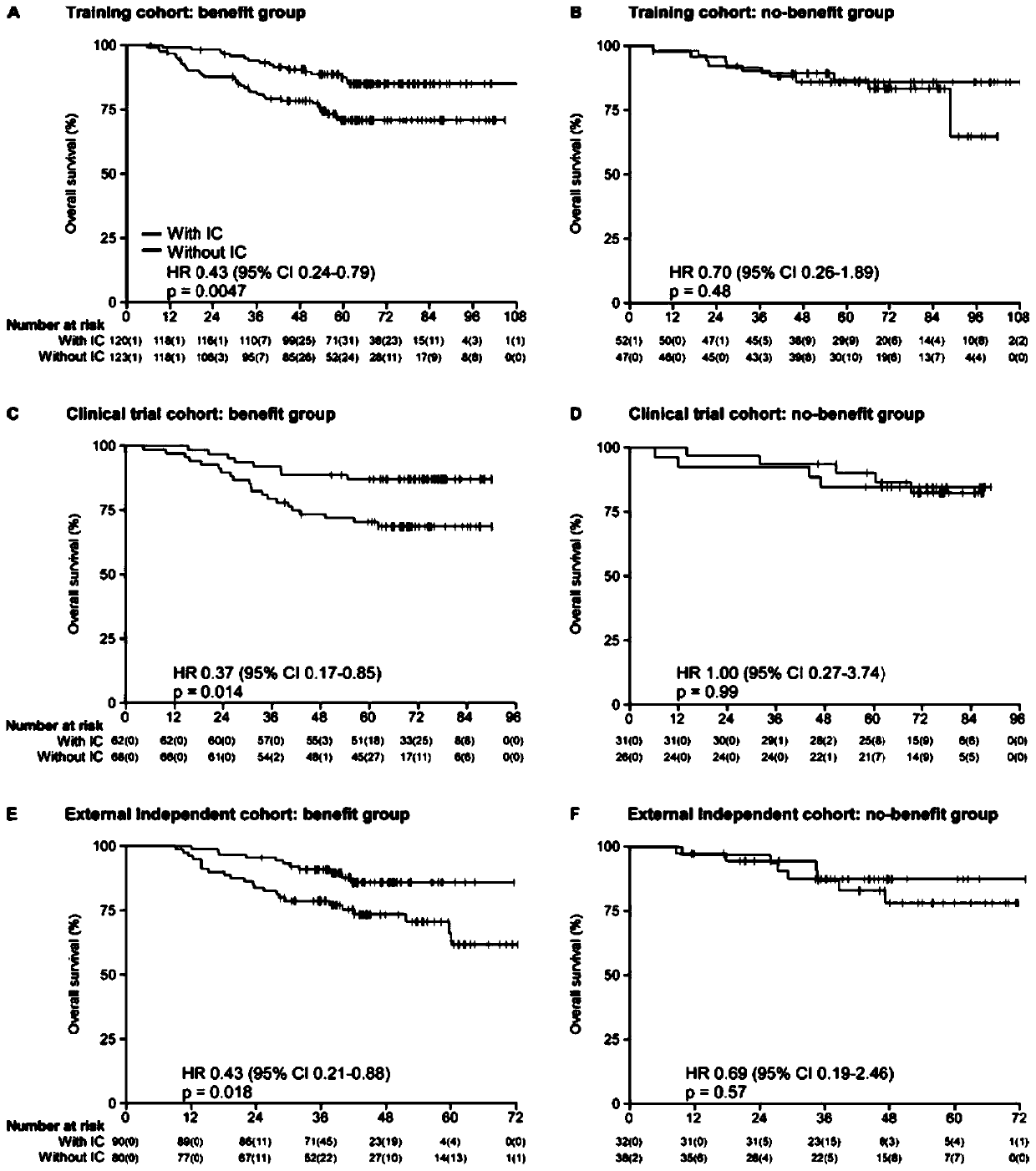
[0036]Of 187 patients in our in-house validation cohort (from a Phase III clinical trial), the gene signature classified 130 (69.5%) patients into benefit and 57 (30.5%) into no benefit ; The AUC of this gene signature to predict the short-term curative effect of nasopharyngeal carcinoma induction chemotherapy reaches 0.82 (95% CI 0.68-0.94), the sensitivity is 87.5%, and the specificity is 71.8% (see appendix figure 1 ); in the benefit group, disease-free survival of patients receiving induction chemotherapy (HR 0.37, 0.18-0.75, P=0.0039; see appendix figure 2 ), overall survival (HR 0.37,0.17-0.85, p=0.014; see appendix image 3 ), distant metastasis-free survival (HR 0.41, 0.18-0.93, p=0.027; see appendix Figure ...
Embodiment 3
[0038] Example 3 Comparison of Molecular Targets for Nasopharyngeal Carcinoma Induction Chemotherapy Efficacy and Other Clinical Indicators Predictive Efficiency
[0039] The inventors compared the effectiveness of the above-mentioned gene signature with other clinical indicators (age, sex, TNM stage, EBV DNA) in predicting the efficacy of induction chemotherapy, and found that the gene signature was equally effective in the training group, internal verification group (clinical trial group) and external verification group. Showed better predictive performance (comparing the ROC of the gene model with other clinical indicators to predict curative effect, all p values were Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com