Chiral diol compound, chiral crown ether compound and preparation methods thereof
A technology of chiral diols and compounds, which is applied in the fields of ether preparation, organic chemical method, and alkylene oxide preparation ether, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
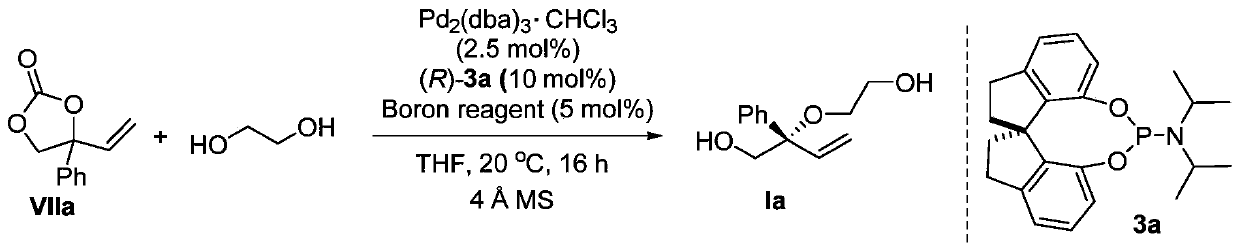
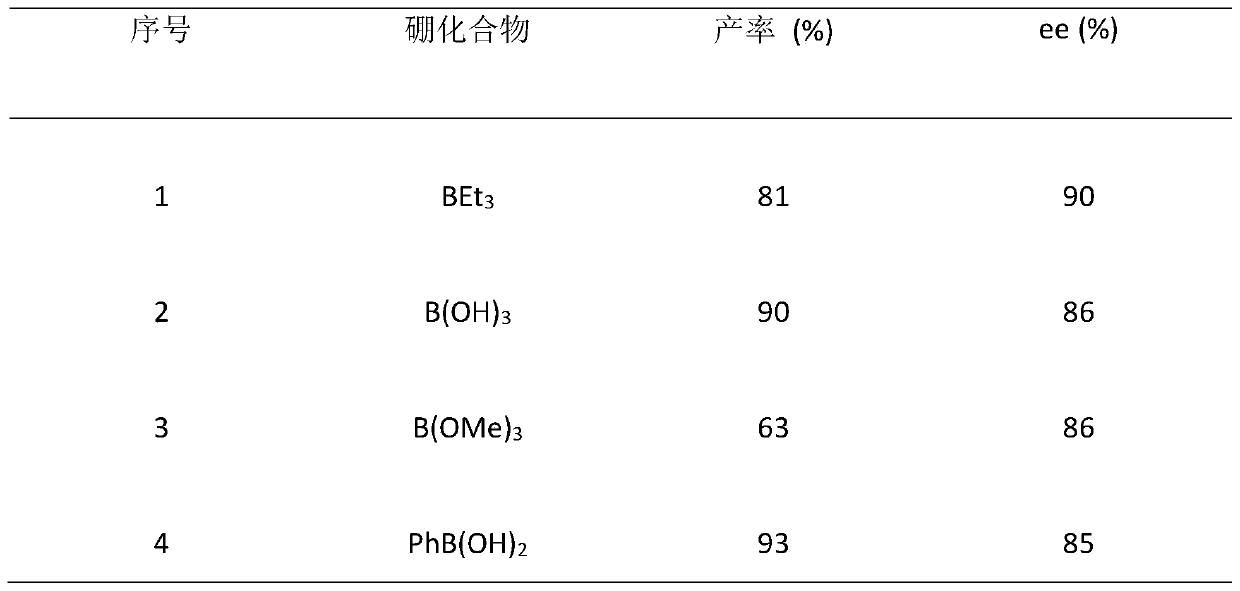
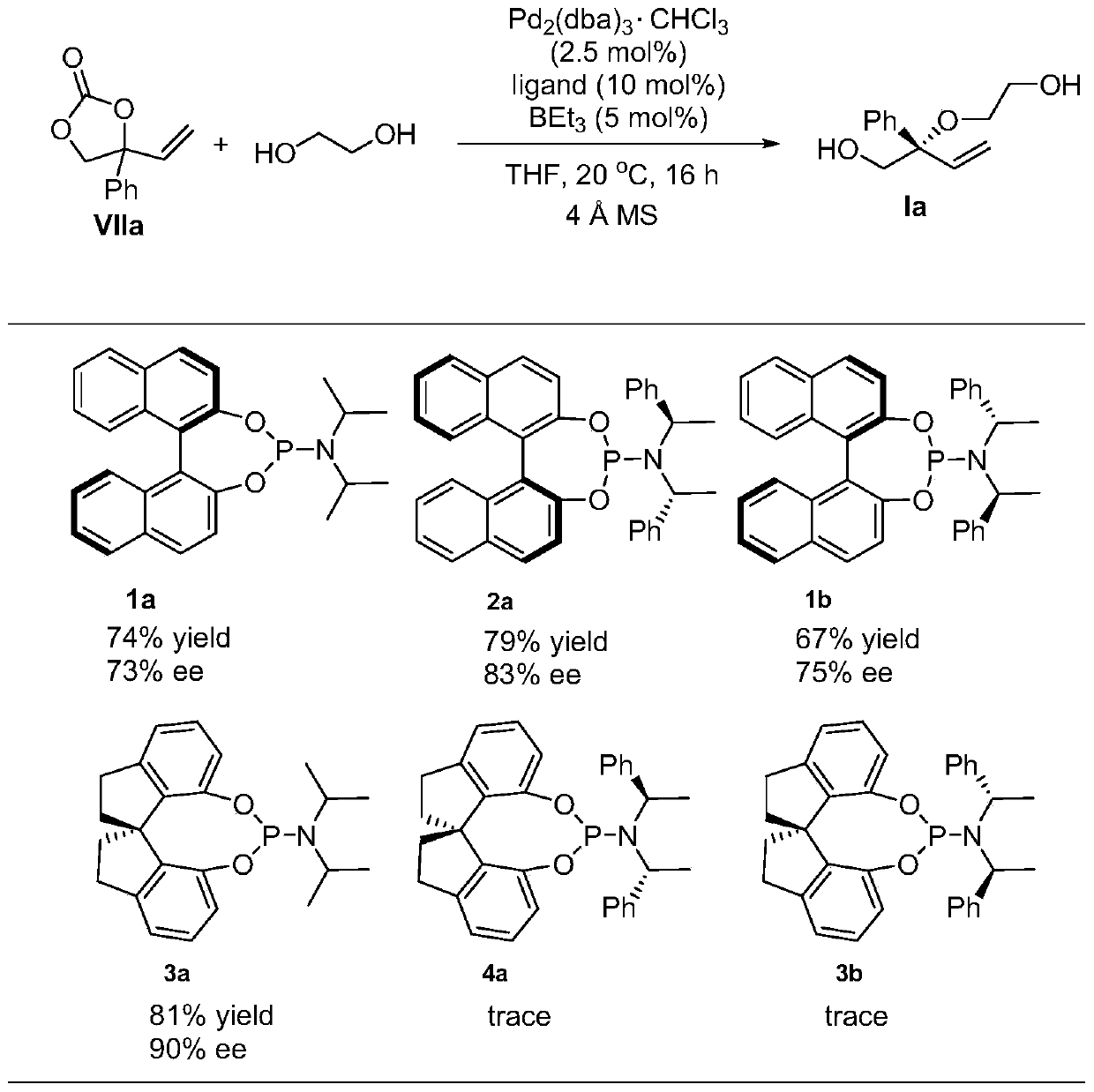
[0043] This embodiment provides a chiral diol compound I, the structural formula of which compound I is as follows:
[0044]
[0045] Among them: R is hydrogen, C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, C 6 -C 26 Arylalkyl, -C n h 2n -OR 1 、-C n h 2n -SR 2 or -C n h 2n -NR 3 R 4 One of them; wherein, n is any integer from 1 to 10, R 1 , R 2 , R 3 and R 4 for hydrogen, C 1 -C 8 Alkyl, C 4 -C 15 Aryl or C 5 -C 15 Arylalkyl;
[0046] R' is -CH 2 CH 2 -(X-CH 2 CH 2 ) m -or-CH 2 -Y-CH 2 -; Wherein, X is O, S or N-R 5 ; 5 for the above R 1 One of them; m is any integer from 0-10000; Y is C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, ...
Embodiment 2
[0051] This embodiment provides a chiral diol compound I, the structural formula of which compound I is as follows:
[0052]
[0053] Among them: R is hydrogen, C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, C 6 -C 26 Arylalkyl, -C n h 2n -OR 1 、-C n h 2n -SR 2 or -C n h 2n -NR 3 R 4 One of them; wherein, n is any integer from 1 to 10, R 1 , R 2 , R 3 and R 4 for hydrogen, C 1 -C 8 Alkyl, C 4 -C 15 Aryl or C 5 -C 15 Arylalkyl;
[0054] R' is -CH 2 CH 2 -(X-CH 2 CH 2 ) m -or-CH 2 -Y-CH 2 -; Wherein, X is O, S or N-R 5 ; 5 for the above R 1 One of them; m is any integer from 0-10000; Y is C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, ...
Embodiment 3
[0059] This embodiment provides a chiral diol compound I, the structural formula of which compound I is as follows:
[0060]
[0061] Among them: R is hydrogen, C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, C 6 -C 26 Arylalkyl, -C n h 2n -OR 1 、-C n h 2n -SR 2 or -C n h 2n -NR 3 R 4 One of them; wherein, n is any integer from 1 to 10, R 1 , R 2 , R 3 and R 4 for hydrogen, C 1 -C 8 Alkyl, C 4 -C 15 Aryl or C 5 -C 15 Arylalkyl;
[0062] R' is -CH 2 CH 2 -(X-CH 2 CH 2 ) m -or-CH 2 -Y-CH 2 -; Wherein, X is O, S or N-R 5 ; 5 for the above R 1 One of them; m is any integer from 0-10000; Y is C 1 -C 20 Alkyl, C 3 -C 16 Cycloalkyl, C 4 -C 16 The heterocyclic group containing N, O or S, C 6 -C 24 The aryl group, the substituent contains N, O, S, P or halogen C 6 -C 24 Substituted aryl, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com