Tumor-related gene FBXW7 mutation-related antigen short-peptide and application thereof
A tumor-related gene and short peptide technology, applied in the field of biomedicine, can solve the problem of weak affinity between CDP and FBXW7, and achieve the effect of good clinical transformation and disease prevention prospects, good application potential, and low difficulty in chemical synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Prediction of T cell epitope of FBXW7 gene mutant peptide and design of FBXW7 short peptide
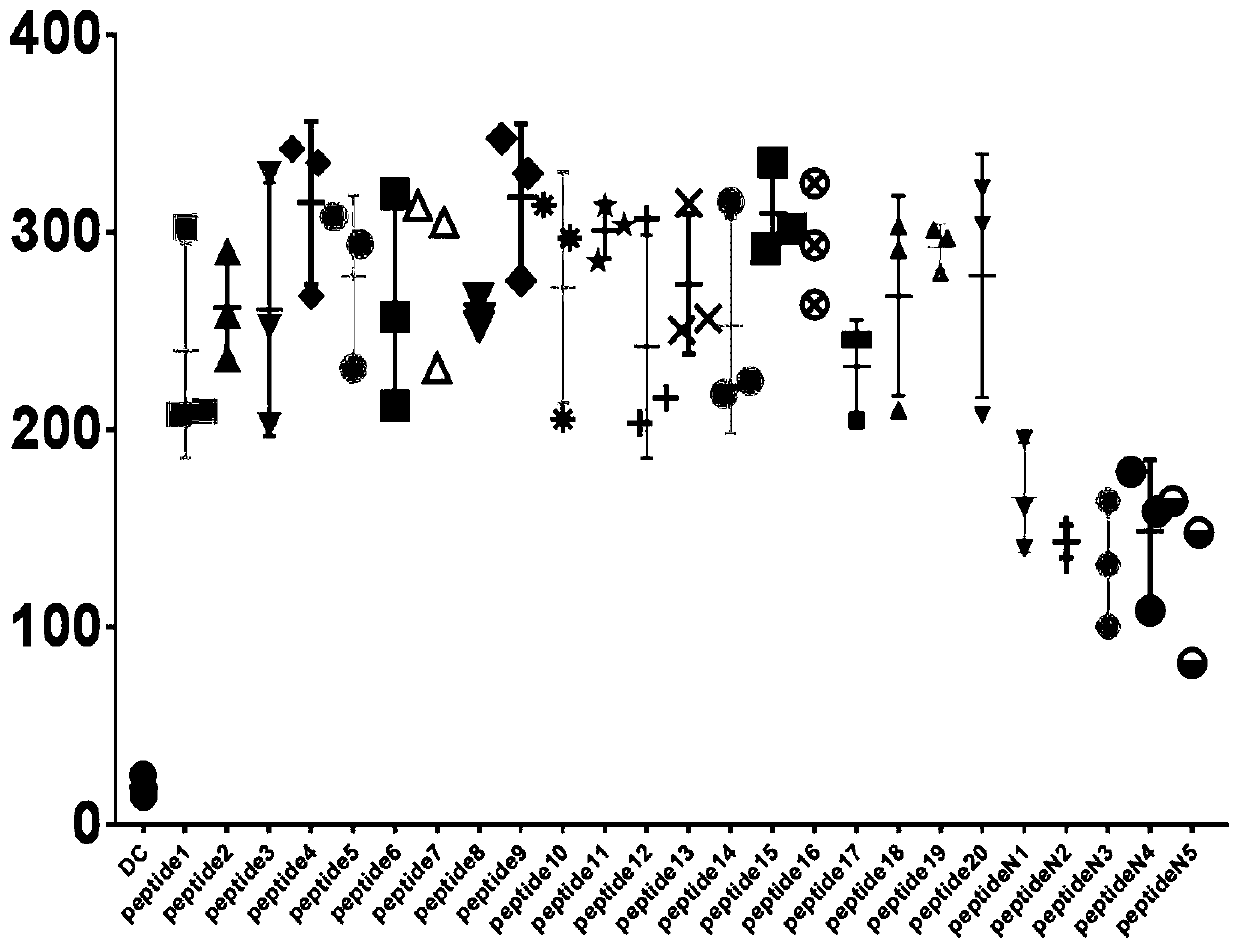
[0043] 1. Through the comprehensive platform of T cell epitope prediction data (http: / / www.cbs.dtu.dk / services / NetCTL) and bioinformatics technology, analyze and predict T cell epitope receptor (TCR) and MHC class I Molecular high-affinity peptide sequences. A series of FBXW7 mutation-related antigen short peptides were obtained through research.
[0044] 2. Screen out 50 polypeptide fragments with high predicted scores and low predicted toxicity from the above polypeptides for further research. The specific experiment is as follows:
[0045] The method for establishing FBXW7 mutant short peptide-specific CTL clones is as follows:
[0046] 10 by Flow Sorting of Healthy Donors 5 CD8 + T cells, added Mo-DCs10 loaded with FBXW7 mutant short peptide 4 Stimulated twice at intervals of 1 week, and then treated with mitomycin C-loaded FBXW7 short peptide autologous per...
Embodiment 2
[0052] Embodiment 2 Inhibition of tumor growth test
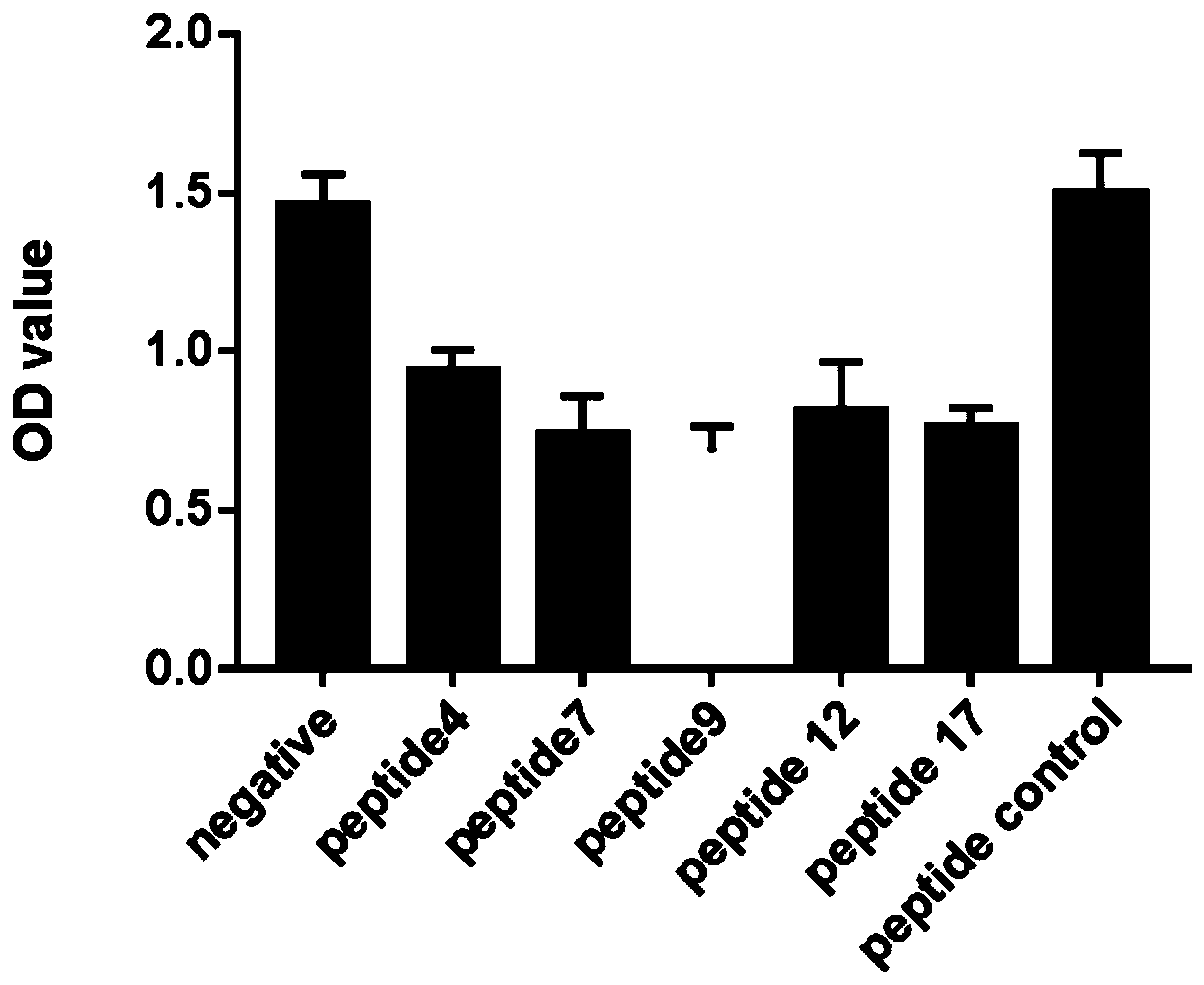
[0053] Example 1 obtained 20 FBXW7 mutant short peptides with specific immune response effects and capable of activating T lymphocytes. Five short peptides were randomly selected from them, and further verification experiments were continued.
[0054] Lung cancer cells A549 were cultured with the cell culture supernatants induced by the 5 short peptides respectively. The control group was divided into two groups: no short peptide loading and nonsense short peptide loading.
[0055] The results showed that, compared with the control group, the supernatants induced by the five short peptides could significantly inhibit the growth of tumor cells and significantly reduce tumor activity (such as figure 2 shown).
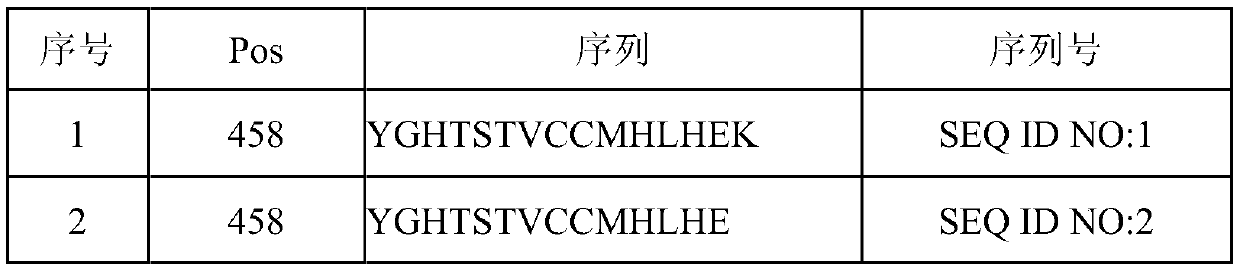
[0056] Experimental results show that the CTL epitope established by the present invention is extremely effective. By presenting the FBXW7 mutant short peptide shown in any one of SEQ ID NO: 1-20 through dendritic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com