Preparation method of tetravalent influenza virus split vaccine
A kind of influenza virus and virus technology, applied in the field of vaccines, can solve the problems of limited protective effect of type B virus, low purity of vaccine antigen, weak cross-protective effect, etc., and achieve the prevention and control of influenza epidemics, good protective effect and wide protection range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
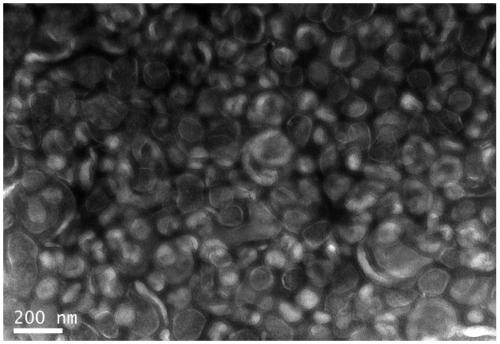
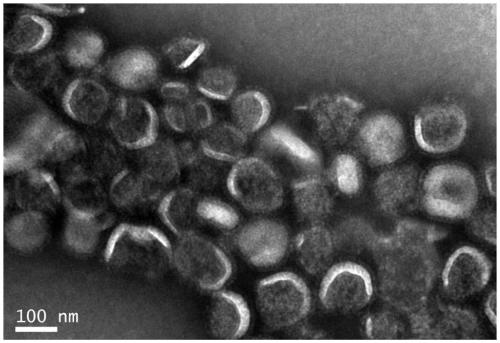
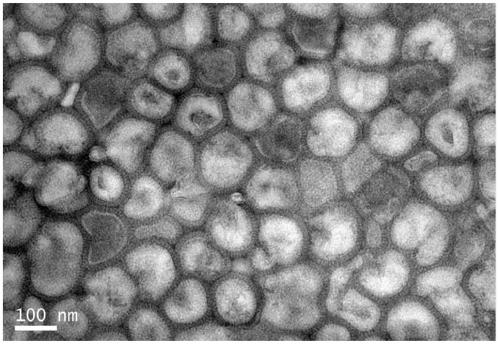
Image
Examples
Embodiment 1
[0065] The preparation method of quadrivalent influenza virus split vaccine of the present invention comprises the steps:
[0066] 1) Harvesting liquid preparation: four influenza (flu) viruses recommended by the World Health Organization and approved by the drug regulatory department of the State Council, H1N1, H3N2, B1 (B / Victoria) and B2 (B / Yamagata) Strains were inoculated with 9-11 day-old healthy chicken embryos respectively, and after 33 35°C, 48 72 hours of culture (48 hours for type A, 72 hours for type B), 2 The virus fluid was harvested after chilling the embryos at 8°C.
[0067] 2) Virus inactivation: add formaldehyde with a final concentration of 200 μg / ml to the monovalent virus pool to inactivate the virus;
[0068] 3) Concentration by ultrafiltration: the inactivated harvest liquid is concentrated by ultrafiltration using a 1000KD ultrafiltration membrane bag to obtain a concentrated virus solution;
[0069] 4) Purification after concentration 1: first a...
Embodiment 2
[0085] Embodiment 2 Carry out control experiment with the vaccine of the preparation of embodiment 1 and comparative example 1
[0086] 1. Detection method of ovalbumin content:
[0087] 1) Before use, equilibrate all reagents to room temperature at room temperature for at least 30 minutes;
[0088] 2) Dilute the sample with the sample diluent, where the reference dilution multiples of the unit price stock solution are original times, 2.5 times, 5 times, and 10 times; the reference dilution multiples of semi-finished products and finished products are original times, 2.5 times, and 5 times; other samples are based on estimates If the measured values of different dilutions are greater than the corresponding absorbance value of the standard 20ng / ml, the sample needs to be further diluted and determined so that the measured value is at the corresponding absorbance value of 0.31-20ng / ml interval;
[0089] 3) Take out the ELISA plate, and add 100 μl of HRP conjugate to each tes...
Embodiment 3
[0118] Example 3 Immune effect test
[0119] Clinical Trial Overview, Immunogenicity Study Endpoints, Immunogenicity Detection Methods
[0120] The S201510001-1 batch of quadrivalent influenza virus split vaccine prepared by the method of Example 1 was tested and qualified by the China Institute for Food and Drug Control before clinical trials. A randomized, double-blind, controlled Phase III clinical trial completed in China In the trial, a total of 3,664 subjects aged 3 and over were enrolled and randomly inoculated with one dose of this product's quadrivalent influenza test vaccine and two trivalent seasonal influenza control vaccines at a ratio of 2:1:1.
[0121] Blood samples were collected from the subjects before and 28 days after immunization, and the influenza virus HI antibody titer (GMT) of the subjects' serum was determined by the micro-hemagglutination inhibition test method.
[0122] The H1N1, H3N2, B(Y) and B(V) antibody GMTs of the whole study population in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com