Crystal form of oxytocin receptor inhibitor and preparation method thereof
A technology of crystal form and solvent, applied in the field of crystal form and preparation of oxytocin receptor inhibitor, can solve problems such as difference in bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Compound 5-(3-(3-(6-fluoronaphthalen-1-yl)azetidin-1-yl)-5-(methoxymethyl)-4H-1,2, Preparation of 4-triazol-4-yl)-2-methoxypyridine
[0098] The first step: tert-butyl 3-(6-fluoro-3,4-dihydronaphthalen-1-yl)azetidine-1-carboxylate 1c
[0099] tert-butyl 3-iodoazetidine-1-carboxylate 1b (1134.58mg, 4.01mmol, prepared by the known method "Organic Letters, 2014, 16(23), 6160-6163"), iodine (39.12mg, 0.15mmol), zinc (604.65mg, 9.25mmol) were added to the reaction flask, protected by argon, and reacted for 0.5 hours. Bis(dibenzylideneacetone)palladium (141.15mg, 0.15mmol), 2-di Cyclohexylphosphine-2′,4′,6′-triisopropylbiphenyl (73.48mg, 0.15mmol) and 4-bromo-7-fluoro-1,2-dihydronaphthalene 1a (700mg, 3.08mmol, using Prepared by the known method "Chemistry-A European Journal, 2015, 21(14), 5561-5583") Add the above reaction solution, after the addition is complete, heat and stir at 50°C for 3 hours. After cooling to , the reaction solution was concentrated under...
Embodiment 2
[0241] Example 2 compound, Example 17 compound, Example 34 compound, Example 37 compound, Example 38 compound, Example 39 compound, Example 42 compound and Example 43 compound.
[0242] 2.2 Test animals
[0243] Twenty-four healthy adult SD rats were divided into 8 groups on average, with 3 rats in each group, purchased from Shanghai Jiesijie Experimental Animal Co., Ltd., animal production license number: SCXK (Shanghai) 2013-0006.
[0244] 2.3 Drug preparation
[0245]Weigh a certain amount of drug, add 2.5% volume of DMSO and 97.5% volume of 10% solutol HS-15 to prepare a 0.2 mg / mL colorless, clear and transparent liquid.
[0246] 2.4 Administration
[0247] SD rats were fasted overnight and administered intragastrically. The dosage was 30.0 mg / kg, and the volume was 10.0 mL / kg.
[0248] 3. Operation
[0249] Rats were administered the compound of Example 2, the compound of Example 17, the compound of Example 34, the compound of Example 37, the compound of Example 38, t...
Embodiment 3
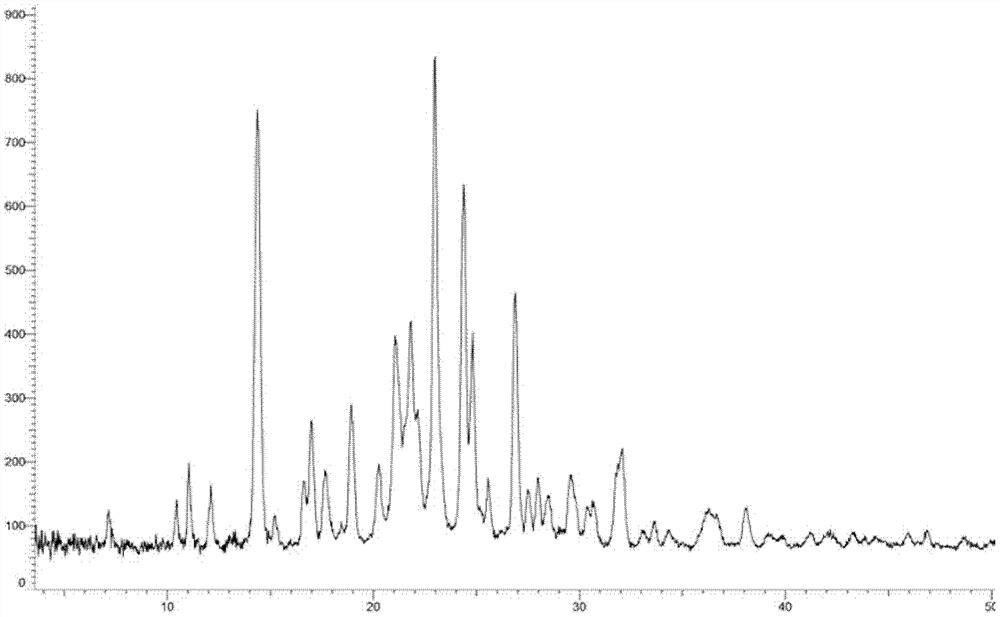
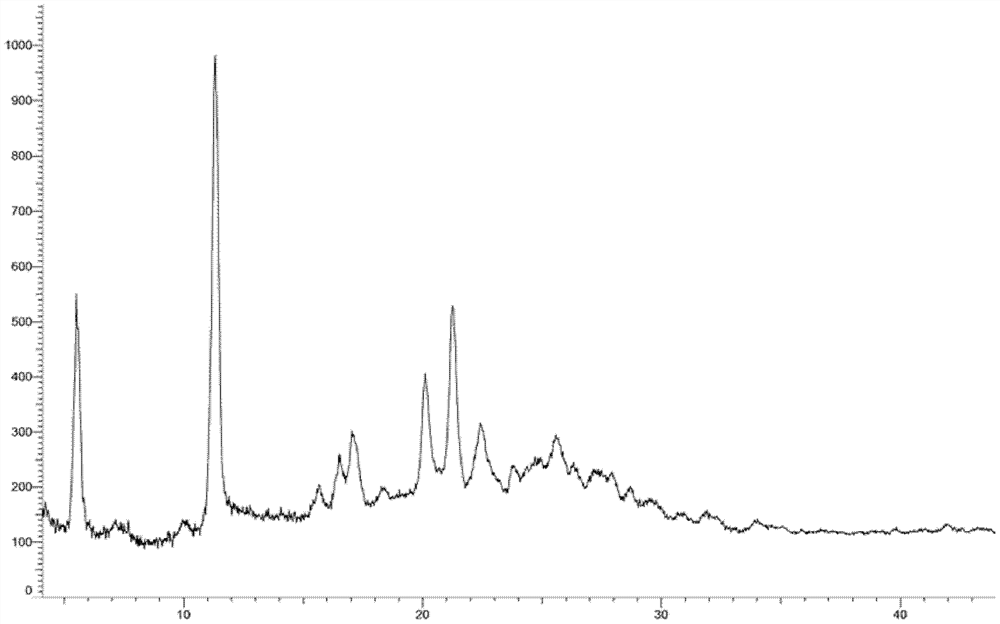
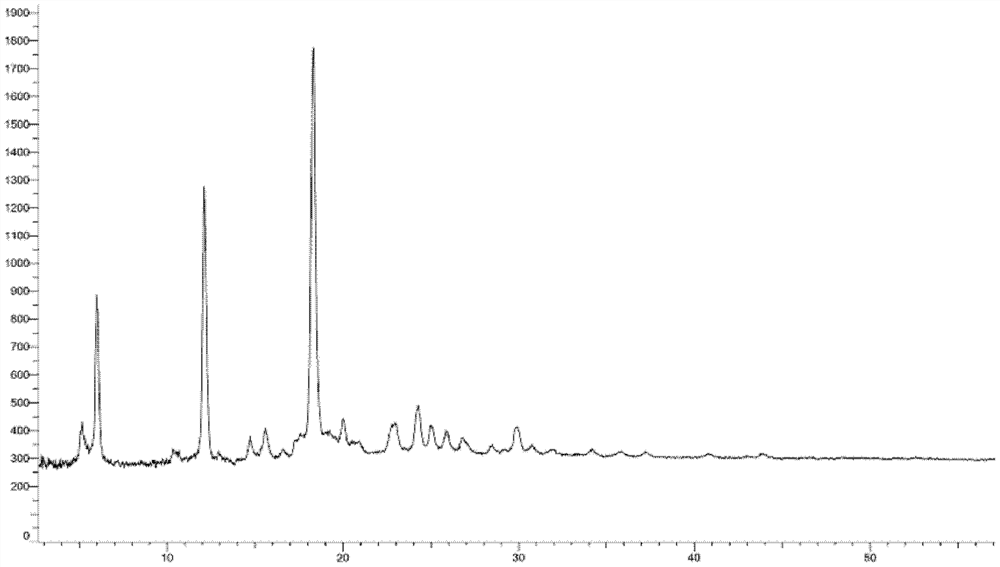
[0260] Embodiment 3: A crystal form
[0261] 1.2g compound 5-(3-(3-(6-fluoronaphthalen-1-yl)azetidin-1-yl)-5-(methoxymethyl)-4H-1,2,4 -Triazol-4-yl)-2-methoxypyridine was added to 8ml ethyl acetate and 24ml n-hexane, heated and stirred to dissolve, stirred and cooled to crystallize, filtered, and vacuum-dried to obtain the product (985mg, yield: 82.1 %).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com