Process for supercritical CO2 hydrogenation based on amorphous alloy
An amorphous alloy and supercritical technology, which is applied in the field of supercritical CO2 hydrogenation to achieve the effects of saving heating costs, strong reaction stability and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Reaction system: 10mL DMF as reaction solvent, add 2.5mL organic amine compound (as organic base, the same below), specifically triethylamine, the purpose of adding organic amine compound is to promote the formation of formate so that the reaction moves to the right . At the same time, 100 mg of NiB amorphous alloy was added as a catalyst. Mix the above systems into the reaction kettle, then empty and fill with gas. For specific implementation steps, please refer to the reaction implementation process.
[0057] Reaction temperature: The reaction temperature is stabilized at 40°C by the temperature control unit.
[0058] Total reaction pressure: 1MPa.
[0059] H in reaction 2 with CO 2 Scale: 0.2.
[0060] Reaction device and instructions for use:
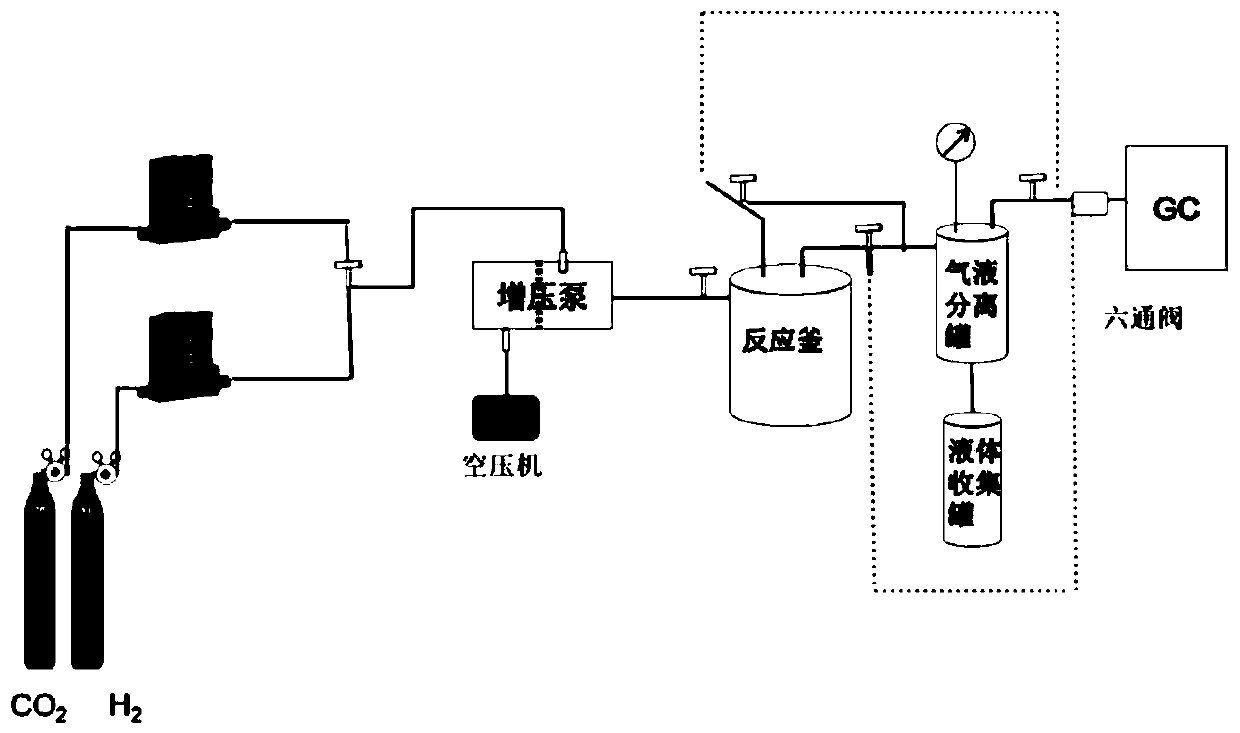
[0061] Such as Figure 1~2 As shown, the reaction was carried out in a set of high-pressure catalytic reactor designed and built by ourselves. The reaction device is composed of a gas circuit control unit, a pressuri...
Embodiment 2
[0066] Reaction system: 10mL DMF was used as the reaction solvent, and 2.5mL TEA was added as the organic base. The purpose of adding the organic base was to promote the formation of formate so that the reaction shifted to the right. At the same time, 100 mg of NiB amorphous alloy was added as a catalyst. Mix the above systems into the reaction kettle, then empty and fill with gas. For specific implementation steps, please refer to the reaction implementation process.
[0067] Reaction temperature: The reaction temperature is stabilized at 40°C by the temperature control unit.
[0068] The total reaction pressures are: 0.1MPa, 1MPa, 3MPa, 5MPa, 7MPa, 9MPa.
[0069] H in reaction 2 with CO 2 Scale: 0.2.
[0070] Schematic diagram of the reaction device and instructions for use:
[0071] With embodiment 1.
[0072] The specific implementation process of the reaction:
[0073] With embodiment 1.
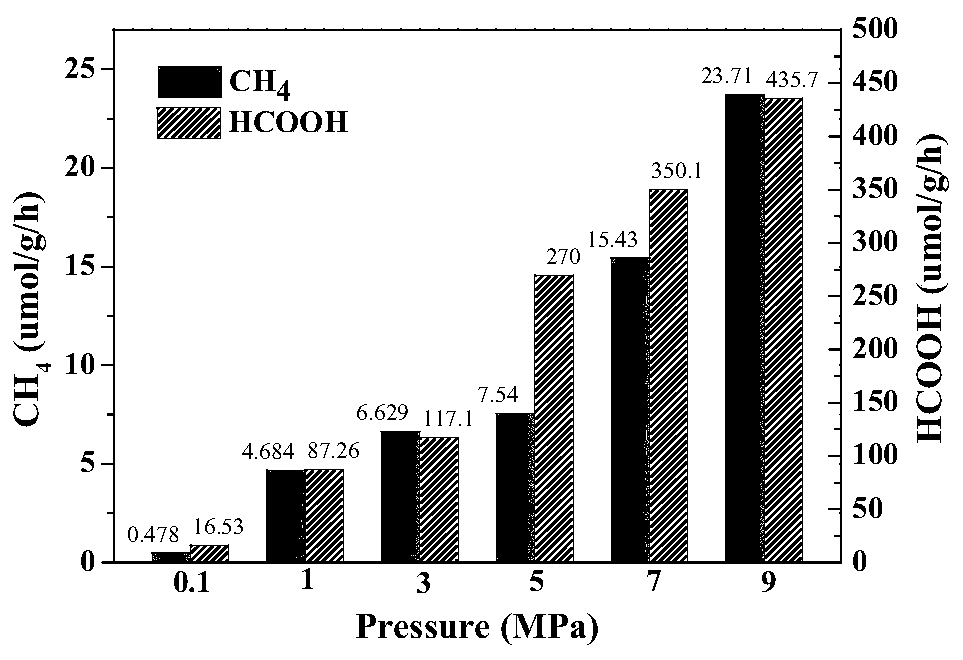
[0074] image 3 It is the activity diagram of the reaction hydrogenation ...
Embodiment 3
[0076] Reaction system: 10mL DMF was used as the reaction solvent, and 2.5mL TEA was added as the organic base. The purpose of adding the organic base was to promote the formation of formate so that the reaction shifted to the right. At the same time, 100 mg of NiB amorphous alloy was added as a catalyst. Mix the above systems into the reaction kettle, then empty and fill with gas. For specific implementation steps, please refer to the reaction implementation process.
[0077] Reaction temperature: The reaction temperature is stabilized at 40°C by the temperature control unit.
[0078] The total reaction pressure is: 9MPa.
[0079] H in reaction 2 with CO 2 The ratios are: 0.1, 0.2, 0.3.
[0080] Schematic diagram of the reaction device and instructions for use:
[0081] With embodiment 1.
[0082] The specific implementation process of the reaction:
[0083] With embodiment 1.
[0084] Figure 4 It is the reaction hydrogenation reaction product CH under different hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com