Detection kit for detecting biomarker of bladder cancer
A technology of biomarkers and diagnostic kits, applied in the fields of biotechnology and medicine, can solve problems such as unsatisfactory sensitivity and specificity, and achieve the effect of avoiding pain and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Separation method of urine exfoliated cells
[0048] Transfer 40ml of human morning urine sample to a 50ml sterile centrifuge tube, centrifuge at room temperature at 2000g for 20min, carefully take out the centrifuge tube, pour off the supernatant slowly, and be careful not to pour off the exfoliated cells in the lower layer. Add 15ml of 1×PBS, invert the centrifuge tube 3-4 times to mix the sample, centrifuge at 2000g room temperature for 20min, carefully take out the centrifuge tube, slowly pour off the supernatant, be careful not to pour off the exfoliated cells in the lower layer, add about 100μl of 1 Resuspend in ×PBS and transfer to a 1.5ml EP tube.
Embodiment 2
[0049] Example 2 Screening of differentially expressed genes
[0050] In order to screen biomarkers related to the diagnosis of bladder cancer, the transcriptome data published in TCGA (https: / / cancergenome.nih.gov / ) and pubmed of NCBI (https: / / www.ncbi.nlm. The candidate markers obtained by consulting relevant literature in nih.gov / pubmed / ) are as follows: CA9, CRH, HOXA13, IGF2, RPS21, CDK1, UBE2C. In addition, at least one of the three internal reference genes SLC25A6, ACTB and GAPDH was selected as an internal reference to normalize the above seven genes.
Embodiment 3
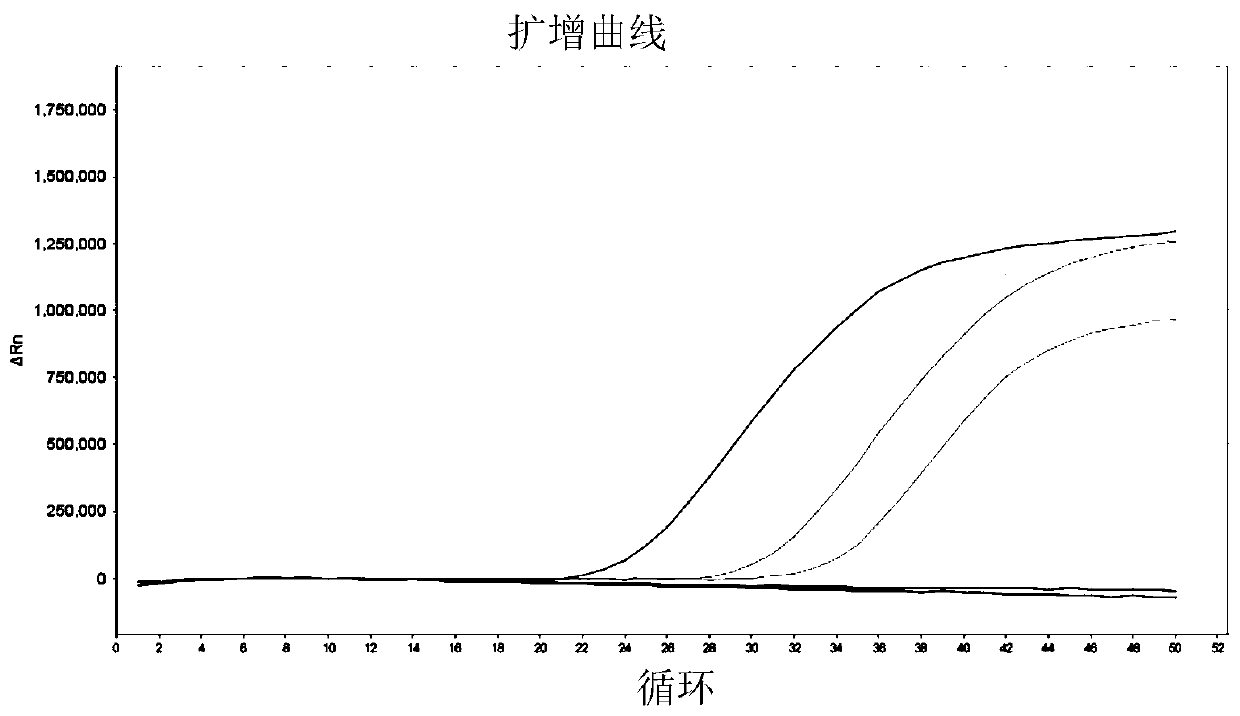
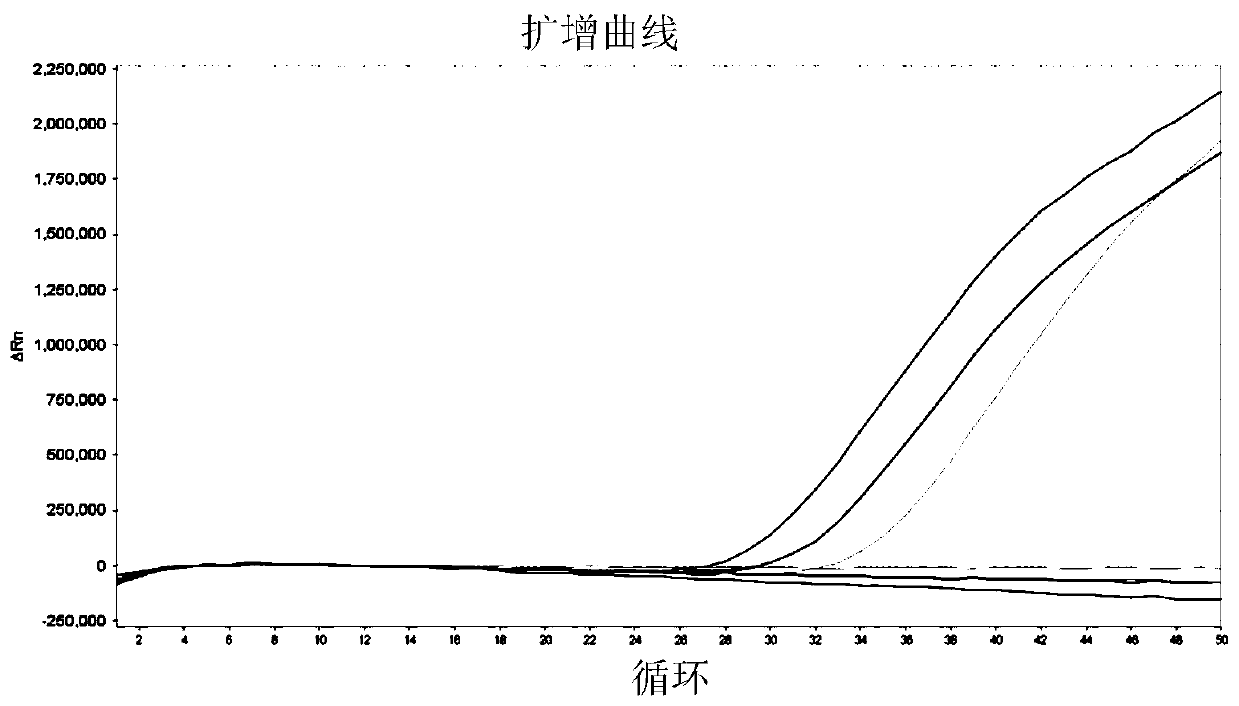
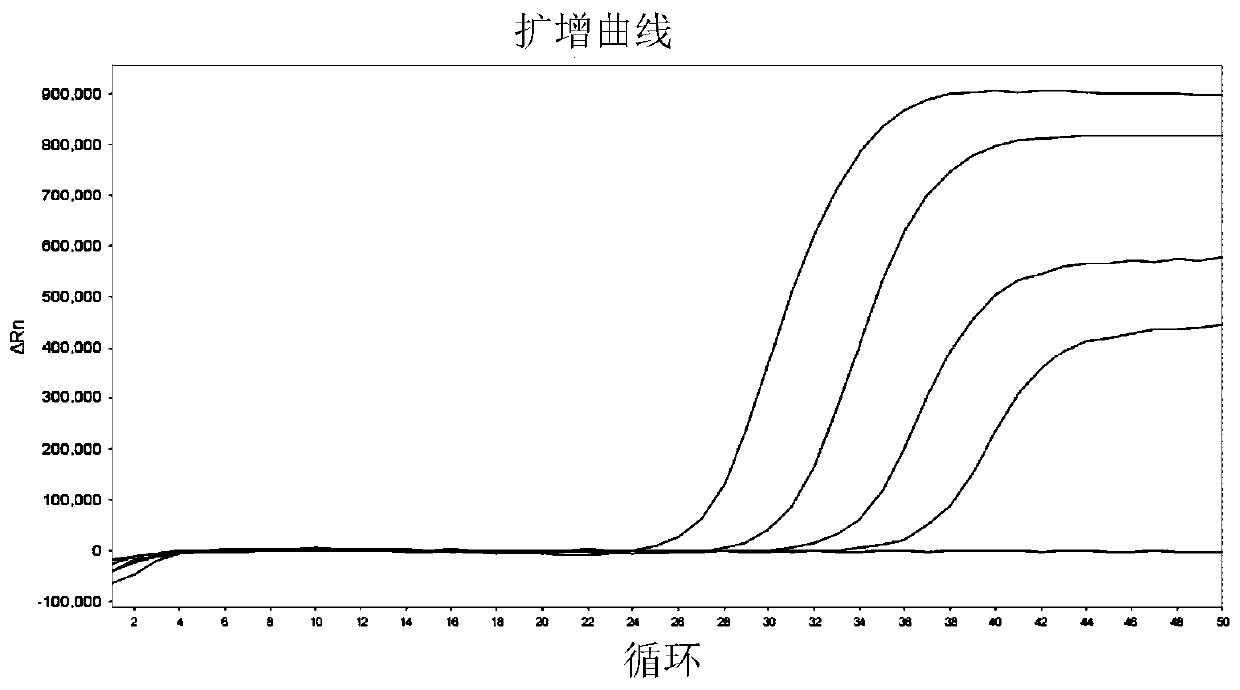
[0051] Example 3 Verify the primers of selected gene markers by real-time fluorescent PCR
[0052] 1. Design and synthesis of primers and probes
[0053] Combining Primer Premier 5 software and Primer-BLAST (NCBI), primers and probes for 7 bladder cancer markers including CA9, CRH, HOXA13, IGF2, RPS21, CDK1 and UBE2C and 3 reference genes including SLC25A6, ACTB and GAPDH were designed . The design principles are as follows: 1) The amplified fragment is less than 150bp; 2) The amplified fragment spans at least one exon; 3) The Tm value of the probe is at least 5°C higher than that of the primer. The designed primers and probe sequences were synthesized by the company, in which the 5' end of the probe was labeled with FAM group, and the 3' end was labeled with BHQ1.
[0054] The nucleotide sequence of the designed primer probe is the sequence shown in SEQ ID NO: 1-30.
[0055] 2. Reverse transcription and qPCR detection
[0056] Takara's PrimeScriptTMRT reagent Kit (Perfect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com