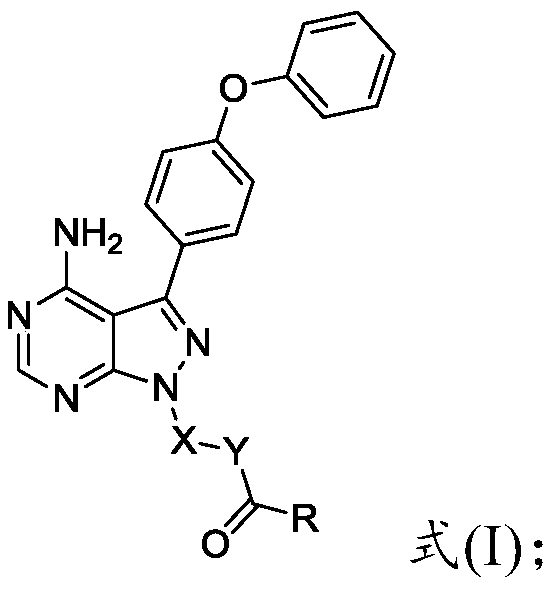
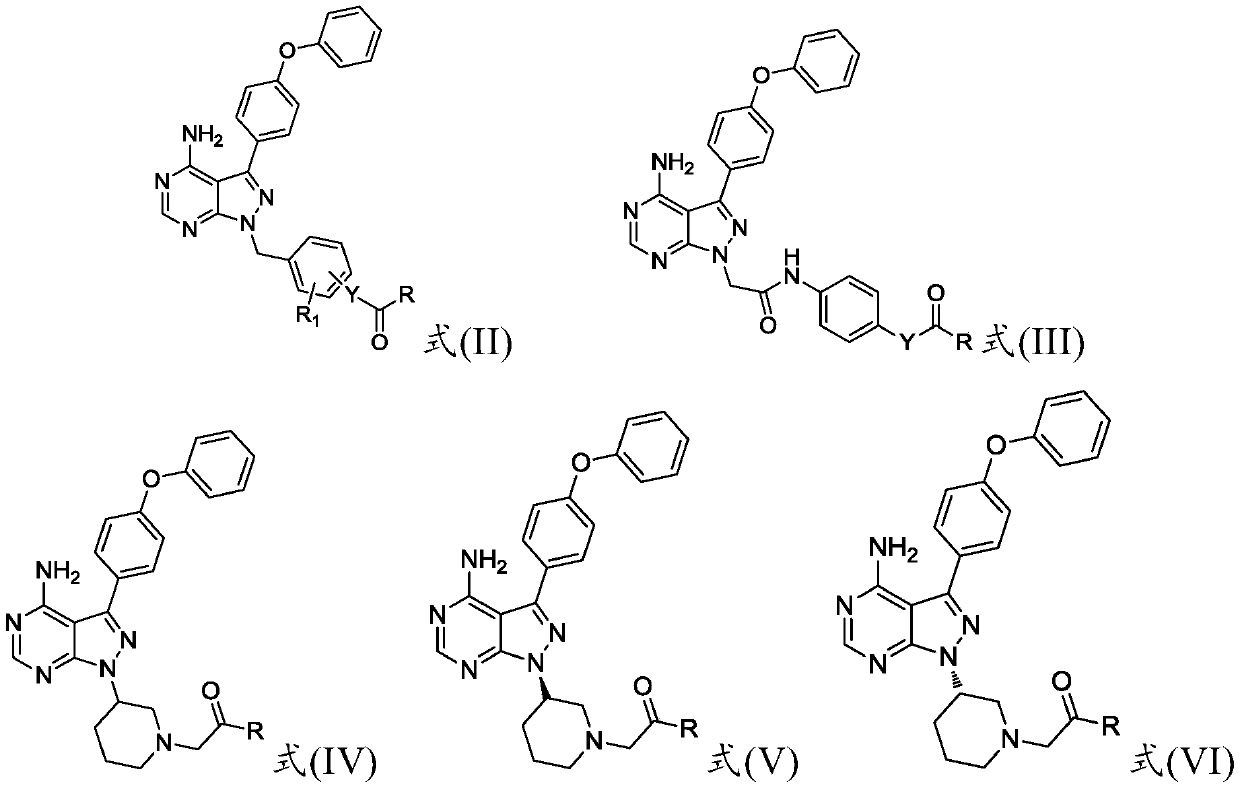
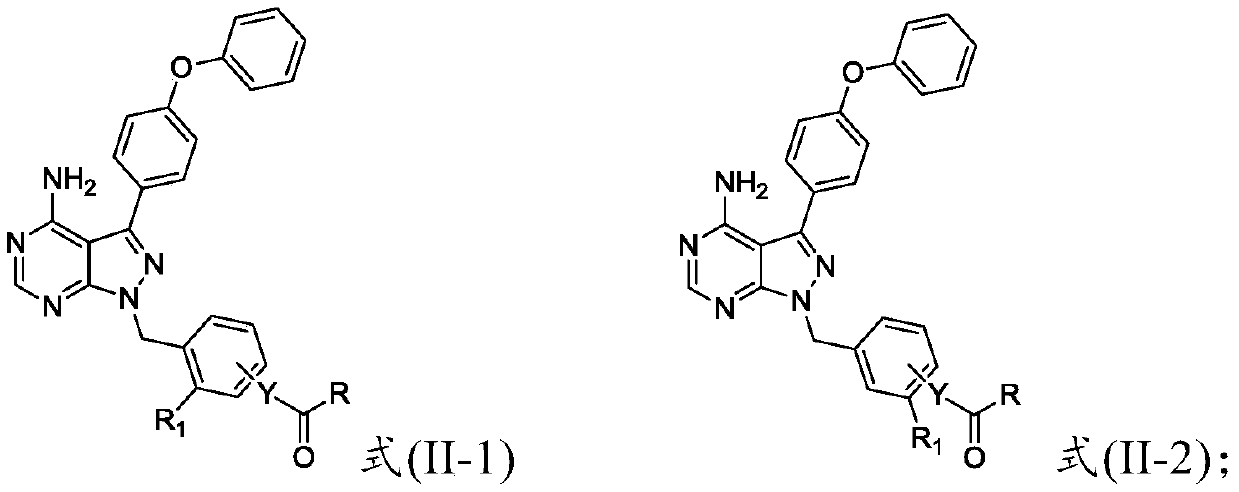
Preparation method and applications of 4-phenoxy phenyl pyrazolopyrimidine amide derivative
A methoxyl and alkoxyl technology, which is used in the synthesis of organic compounds and medical applications, can solve problems such as limited effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The preparation of embodiment 1, intermediate 2
[0116] Dissolve 4-aminopyrazolo[3,4-d]pyrimidine (5g, 37mmol) in 30ml of DMF, add N-bromosuccinimide (7.9g, 44.4mmol), stir, 80°C oil bath Heating, the color of the solution changed from light yellow to red, reacted for 3 hours, TLC detection, the basic reaction was complete, cooled to room temperature, poured the reaction solution into 300ml ice water, stirred, a large amount of yellow solid was precipitated, filtered, and the filter cake was washed with ice water , and dried to obtain 6.33 g of a light yellow solid with a yield of 79.9%. MP: 270-273°C 1 H NMR (400MHz, DMSO) δ13.77(s, 1H), 8.18(s, 1H).
Embodiment 2
[0117] Embodiment 2, the preparation of intermediate 3
[0118] Take 3-bromo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2g, 9.34mmol), 4-phenoxyphenylboronic acid (3.99g, 18.68mmol), tetrakis(triphenylphosphine ) Palladium (531mg, 0.46mmol), K 3 PO 4 ·3H 2 O (7.45g, 28.02mmol), added to a 250ml two-neck bottle, added 100ml of solvent (1,4-dioxane: water = 4:1) to dissolve, nitrogen protection, ultrasonic removal of oxygen in the solution, and nitrogen The air in the replacement device was heated to reflux in an oil bath at 135° C. After 30 hours of reaction, TLC detected that the reaction was basically complete. The reaction solution was cooled to room temperature, filtered with diatomaceous earth, and the filtrate was evaporated under reduced pressure to obtain a yellow solid. Silica gel column chromatography (dichloromethane:methanol=100:1) gave 2.23 g of a light yellow solid with a yield of 78.8 %. MP: 251-254°C 1 H NMR (400MHz, DMSO) δ13.57(s, 1H), 8.22(s, 1H), 7.67(d, J=7...
Embodiment 3
[0119] Embodiment 3, the preparation of intermediate 4
[0120] 1. Preparation of Intermediate 4a
[0121] Take 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (250mg, 0.82mmol), methyl 4-(bromomethyl)benzoate (224mg , 0.98mmol), K 2 CO 3 (170mg, 1.23mmol) and KI (13mg, 0.08mmol) were added to a 50ml reaction bottle, 15ml of DMF was added to dissolve and stirred, and reacted at room temperature for 8 hours, and TLC detected that the reaction was complete. The reaction solution was poured into 100ml of ice water, stirred, and a white solid was precipitated. Added 30ml of ethyl acetate, extracted three times with a separatory funnel, combined the organic phases, washed with saturated brine, evaporated the organic phases under reduced pressure, and performed silica gel column chromatography (dichloro Methane:methanol=200:1), 287 mg of white solid was obtained, and the yield was 77.6%. 1 H NMR (400MHz, DMSO) δ8.28(s, 1H), 7.66(d, J=6.8Hz, 2H), 7.45–7.41(m, 2H), 7.25–7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com