Compound CYD19 or pharmaceutically acceptable salt thereof as Snail inhibitor, and preparation method, pharmaceutical composition and application of compound CYD19 or pharmaceutically acceptable salt thereof
A technology of inhibitors and compounds, applied in drug combinations, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve the problems of uncertain mechanism research, limited compound development, no obvious inhibitory effect, etc., to reduce the level of acetylation , accelerated degradation, significant anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: The preparation method of the compound CYD19 or its pharmaceutically acceptable salt as a Snail inhibitor in this embodiment, the preparation steps are as follows:
[0038]4-[[[4-[(5-methyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]sulfur ]Methyl]benzoic acid is completely dissolved in N,N-dimethylformamide, then add TBTU and N,N-diisopropylethylamine, stir at room temperature and then add p-fluoro-o-phenylenediamine, continue stirring at room temperature , the reaction solution was evaporated to dryness under reduced pressure to obtain the evaporated product, the mixture of the evaporated product and silica gel was added to ethyl acetate solvent, and then evaporated to dryness at a temperature of 40°C to 50°C to obtain the evaporated product, and the evaporated product was added to the upper layer of the silica gel column , and then use petroleum ether and ethyl acetate to elute, the collected eluate is evaporated to dryness under reduced pr...
Embodiment 2
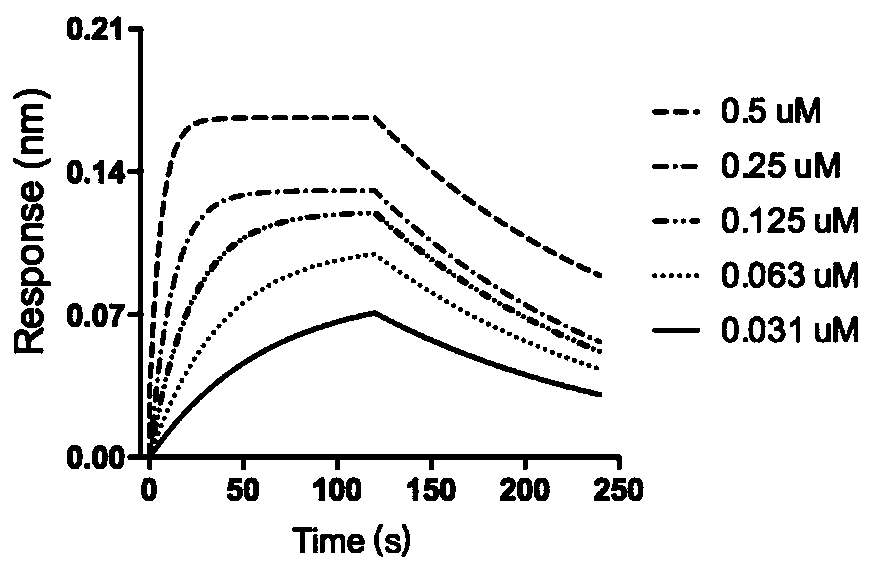
[0039] Example 2: Biolayer interferometry detects the affinity of compound CYD19 to Snail protein.
[0040] Dissolve the recombinant Snail protein in PBS and place it in a 100uL tube. Incubate EZ-Link NHS-biotin and Snail recombinant protein at room temperature for 60 minutes (the molar ratio of protein to biotin is 1:3). Desalting is used to remove excess biotin. The biotinylated proteins were immobilized on Super Streptavidin biosensors for further measurements. Blank recombinant protein was used as a control. CYD19 with different concentrations (0.5, 0.25, 0.125, 0.063, 0.031 μM) was used as a non-specific control. Binding of CYD19 to Snail-protein was determined using an Octet red 96 instrument (ForteBio), the intensity of the light deviation was recorded, and the association and dissociation rates were calculated using a 1:1 binding model. The results showed that CYD19 had a higher affinity with Snail protein (K d = 180nM), figure 1 .
Embodiment 3
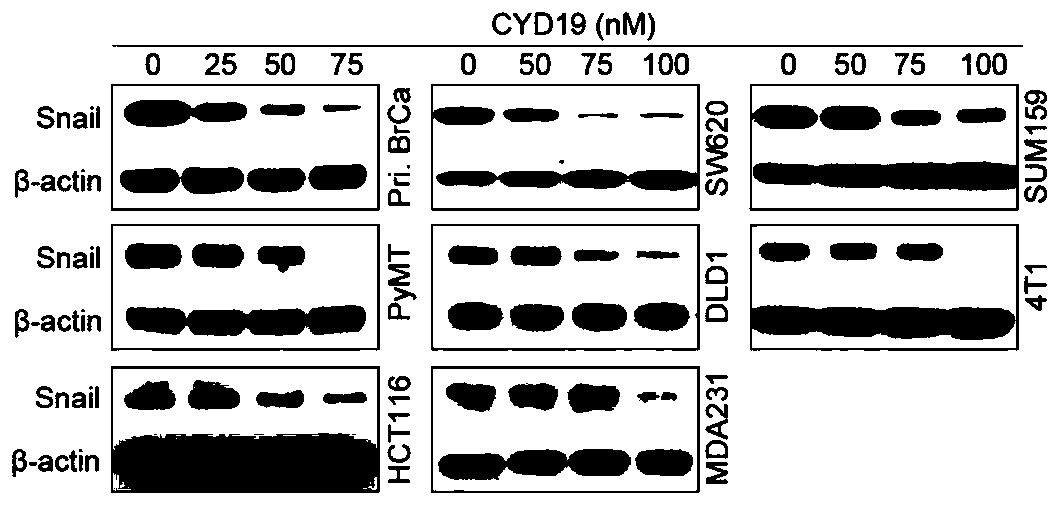
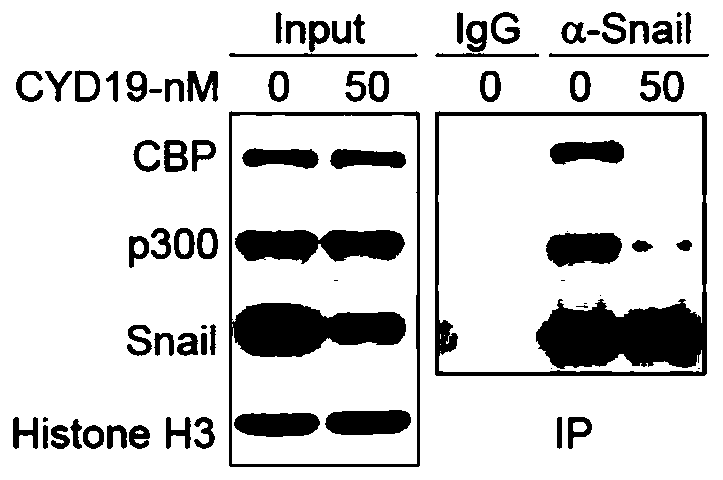
[0041] Example 3: Detection of the effect of CYD19 on the expression of Snail protein by Western Blot.
[0042] The cells were seeded in a six-well plate, and after the cells adhered to the wall for 12-24 hours, they were treated with different concentrations of CYD19 (25, 50, 75, 100 nM) for 24 and 48 hours. After the protein was extracted and the concentration was determined, according to the molecular weight of the protein, Prepare 10% SDS polyacrylamide gel without concentration gradient, 90V stacking gel, 120V separating gel electrophoresis, 2.5-3h. Transfer the membrane through the full wet transfer method, and cut the appropriate size filter paper and nitrocellulose membrane according to the size, glue it on the negative electrode, and the film on the positive electrode, with a constant current of 250mA, and adjust the transfer time according to the required protein Kd number . Immerse the protein-transferred nitrocellulose membrane in Ponceau staining for 5 minutes, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com