Method for detecting purity of protein sample containing polyethylene glycol by using capillary electrophoresis technology
A capillary electrophoresis, polyethylene glycol technology, applied in the preparation of test samples, material inspection products, measuring devices, etc., can solve the problem of little optimization experience and technology, no suitable capillary electrophoresis analysis method, affecting the purity and accuracy of protein samples. Analysis and other problems to achieve the effect of preventing fragmentation and insufficient denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
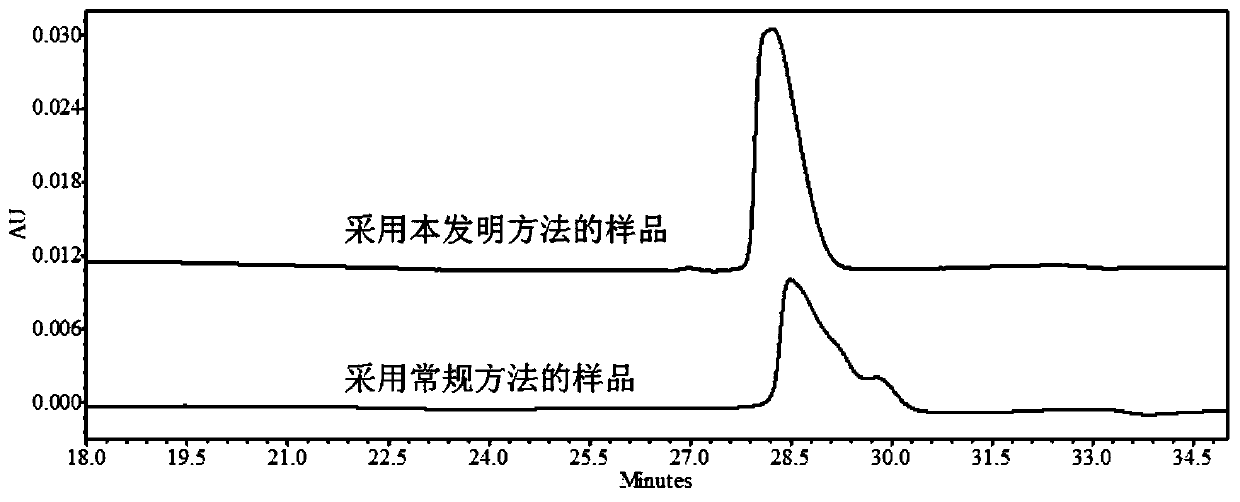
Embodiment 1
[0048] Preparation of buffer solution: Take disodium hydrogen phosphate and citric acid solid powder, add pure water to prepare a buffer solution containing 15.8mmol / L disodium hydrogen phosphate and 4.9mmol / L citric acid.
[0049] The method of the present invention: the protein sample to be analyzed is Herceptinumab solution (21 mg / mL), and the final concentration of polyethylene glycol 8000 is set to 2% (P=2). Substitute the P value and the HMW value (HMW=0.15) of Herceptinumab solution (21 mg / mL) without PEG into HMW=-110.1+1.576T+21.1U+139.12P-0.296T×U In the model of -2.058T×P-30.75U×P+0.4502T×U×P, the value of T should be as low as possible, and the final concentration of urea required was calculated using Minitab software: 3mol / L, and the incubation temperature was: 64.5°C.
[0050] Add the corresponding mass of polyethylene glycol 8000, urea, and sodium lauryl sulfate to the protein sample, and use buffer solution to adjust the total volume of the protein sample to 10...
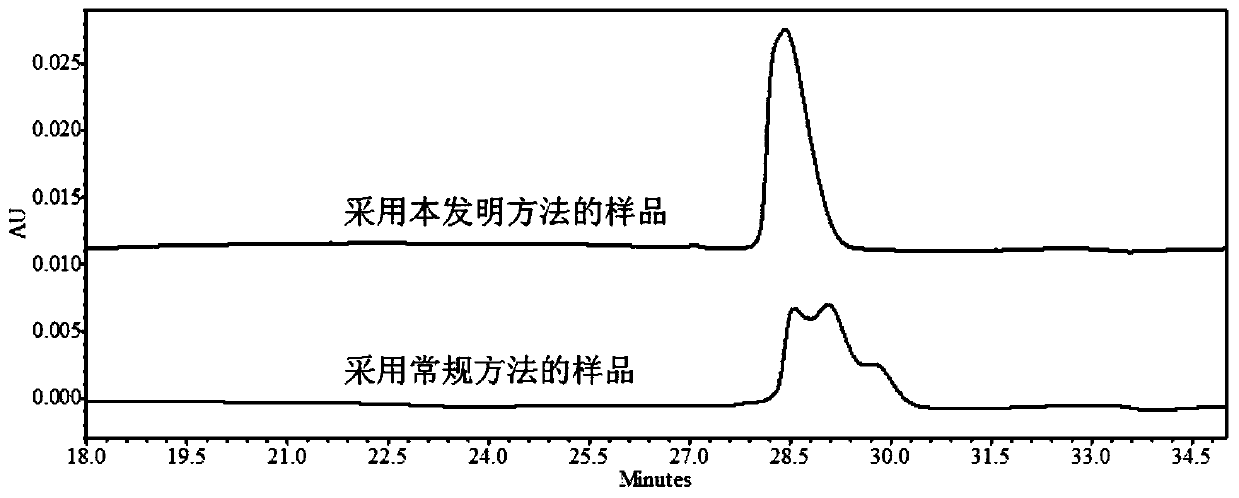
Embodiment 2
[0056] Preparation of buffer solution: Take disodium hydrogen phosphate and citric acid solid powder, add pure water to prepare a buffer solution containing 15.8mmol / L disodium hydrogen phosphate and 4.9mmol / L citric acid.
[0057] The method of the present invention: the protein to be analyzed is Herceptinumab solution (21 mg / mL), and the final concentration of polyethylene glycol 8000 is set to 4% (P=4). Substitute the P value and the HMW value (HMW=0.15) of Herceptinumab solution (21 mg / mL) without PEG into HMW=-110.1+1.576T+21.1U+139.12P–0.296T×U In the model of –2.058T×P–30.75U×P+0.4502T×U×P, T should be as low as possible, and the final concentration of urea required was calculated using Minitab software: 3mol / L, and the incubation temperature was: 65.0°C.
[0058] Add the corresponding mass of polyethylene glycol 8000, urea and sodium lauryl sulfate to the sample and use a buffer solution to adjust the total sample volume to 100 μL and adjust the concentration of Hercep...
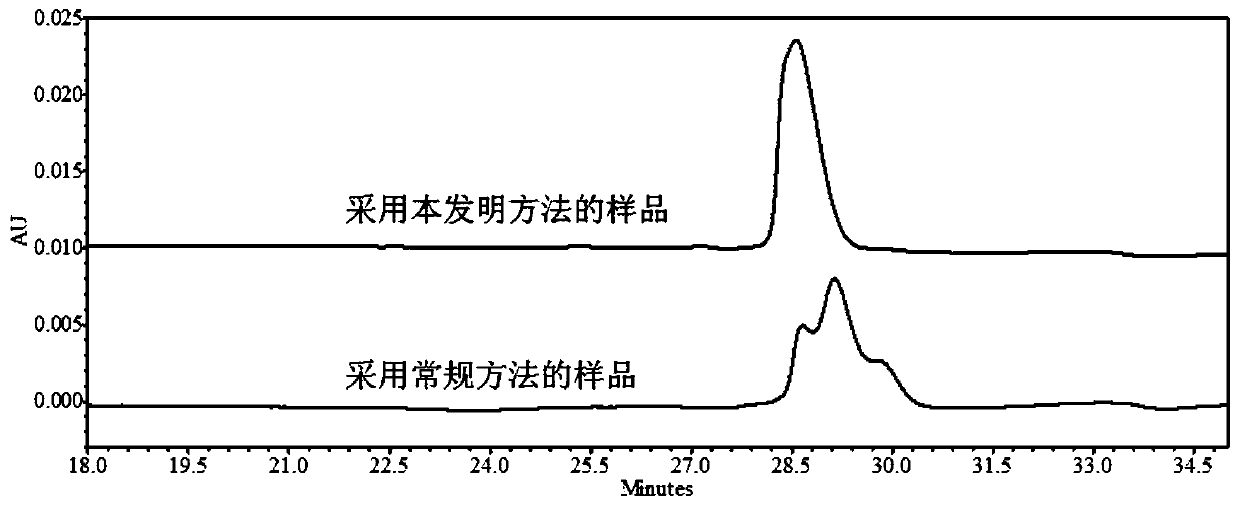
Embodiment 3
[0064] Preparation of buffer solution: Take disodium hydrogen phosphate and citric acid solid powder, add pure water to prepare a buffer solution containing 15.8mmol / L disodium hydrogen phosphate and 4.9mmol / L citric acid.
[0065] The method of the present invention: the protein to be analyzed is Herceptinumab solution (21 mg / mL), and the final concentration of polyethylene glycol 8000 is set to 6% (P=6). Substitute the P value and the HMW value (HMW=0.15) of Herceptinumab solution (21 mg / mL) without PEG into HMW=2954–94.3T–89.1U+112.1P+0.7489T×T+ In the 1.304T×U–1.659T×P model, T should be as low as possible, and the required final concentration of urea was calculated using Minitab software: 3mol / L, and the incubation temperature was: 65.6°C.
[0066] Add the corresponding mass of polyethylene glycol 8000, urea and sodium lauryl sulfate to the sample and use a buffer solution to adjust the total sample volume to 100 μL and adjust the concentration of Herceptin to 1 mg / mL, po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com