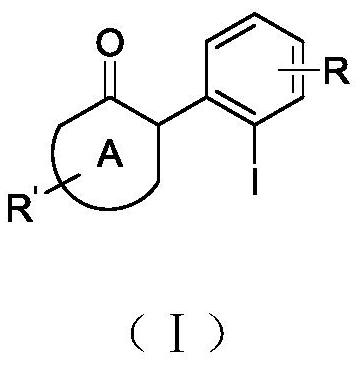
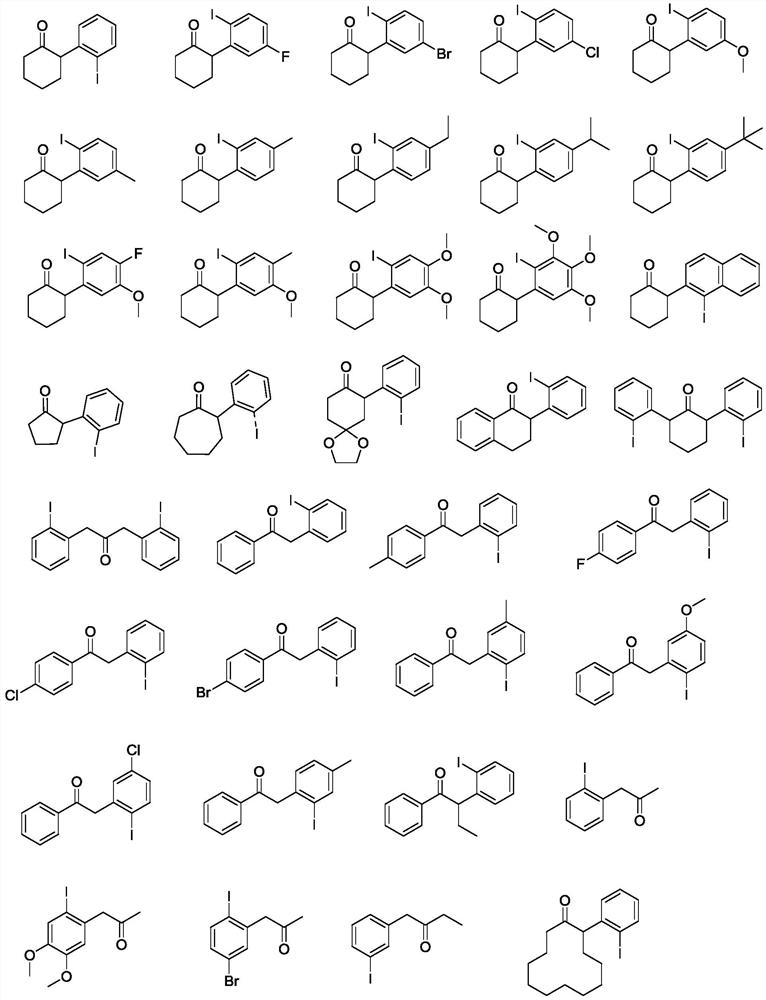
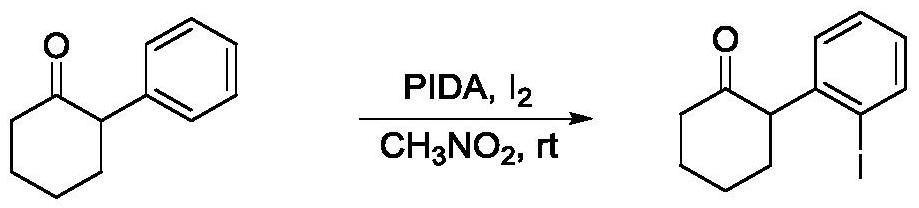A kind of aromatic ring ortho iodoalkanone and its synthesis method and application
A synthetic method and technology of iodoalkanone, which is applied in the field of aromatic ring ortho-iodoalkanone and its synthesis, can solve the problems that cannot be synthesized by intermediates, difficult to stop iodo reaction, difficult iodo synthesis, etc., to achieve Safe and easy to operate, high synthetic yield, suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Using 2-phenylcyclohexanone as raw material
[0027]
[0028] In the 10mL reaction tube, add 2-phenylcyclohexanone (34.8mg, 0.2mmol), PIDA (96.6mg, 0.3mmol) and I 2 (50.8mg, 0.2mmol), then add 1.0mL nitromethane, react at room temperature for 2.5h. After the reaction of the raw materials is complete, add a saturated aqueous solution of sodium thiosulfate to the system to quench the remaining oxidant, extract with DCM (5mL*3), wash with water (5mL*3), wash with saturated brine (5mL*2), and perform column chromatography directly (petroleum ether / ethyl acetate=30:1). A colorless liquid (43.2 mg, yield 72%) was finally obtained.
[0029] The product detection data are as follows:
[0030] 1 H NMR (400MHz, CDCl 3 ): δ7.84(dd, J=7.9Hz, J=1.2Hz, 1H), 7.34(td, J=7.5Hz, J=1.2Hz, 1H), 7.17(dd, J=7.8Hz, J=1.7 Hz, 1H), 6.95(td, J=7.8Hz, J=1.7Hz, 1H), 3.99(dd, J=12.3Hz, J=5.2Hz, 1H), 2.57-2.54(m, 2H), 2.31- 2.27(m, 1H), 2.22-2.19(m, 1H), 2.06-2.03(m, 1H), 1.97-1.81(m, 3H);...
Embodiment 2
[0032] Using 2-phenylcyclohexanone as raw material
[0033]
[0034] 2-Phenylcyclohexanone (34.8mg, 0.2mmol, PIFA (129mg, 0.3mmol) and I2 (50.8mg, 0.2mmol) were sequentially added into a 10mL reaction tube, and then 1.0mL HFIP was added, and reacted at room temperature for 7h. The reaction of the raw materials was complete, adding a saturated aqueous solution of sodium thiosulfate to the system to quench the remaining oxidant, extracting with DCM (5mL*3), washing with water (5mL*3), washing with saturated brine (5mL*2), and directly performing column chromatography ( Petroleum ether / ethyl acetate=30:1).A colorless liquid (46.8 mg, yield 78%) was finally obtained.
[0035] The spectral data of the product are the same as in Example 1.
Embodiment 3
[0037] Using 2-phenylcyclohexanone as raw material
[0038]
[0039] 2-Phenylcyclohexanone (34.8mg, 0.2mmol), IBX (84mg, 0.3mmol)) and I2 (50.8mg, 0.2mmol) were sequentially added to a 10mL reaction tube, and then 1.0mL HFIP was added, and reacted at room temperature for 4d . After the raw material completely disappeared, add a saturated aqueous solution of sodium thiosulfate to the system to quench the remaining oxidant, extract with DCM (5mL*3), wash with water (5mL*3), wash with saturated brine (5mL*2), and perform column chromatography directly (petroleum ether / ethyl acetate=30:1). A colorless liquid (46.2 mg, yield 77%) was finally obtained.
[0040] The spectral data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com