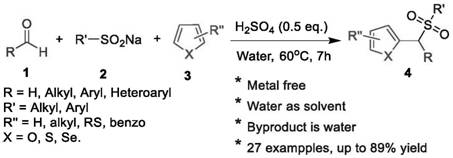
A kind of synthetic method of aryl (chalcogen heteroaryl) methyl sulfone
A synthetic method and a heteroaryl technology are used in the synthesis field of aryl (chalcogenheteroaryl) methyl sulfone, and can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
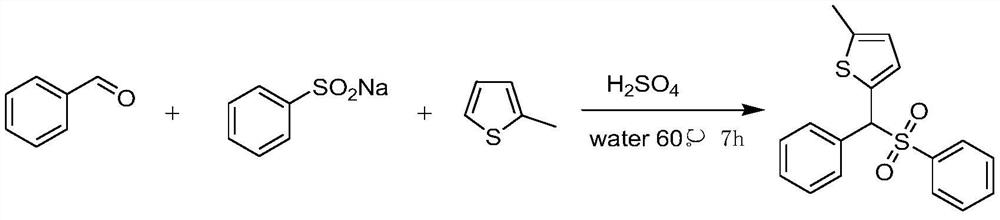
[0011] To a 25 mL glass test tube equipped with a stir bar was added benzaldehyde (1.5 mmol), 2-methylthiophene (1 mmol), sulfuric acid (0.5 equiv) and 2 mL of water. The tube was stirred in a preheated oil bath at 60°C for 15 minutes, then sodium benzenesulfinate (1 mmol) was added slowly. The reaction mixture was stirred at 60°C. After 7 hours, the progress of the reaction was checked by TLC and confirmed that the reaction was complete. The reaction mixture was cooled to room temperature. Water (10 mL) was then added to the reaction mixture, which was extracted three times with ethyl acetate (10 mL). The combined organic layers were washed with anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure. The residue was purified by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 10:1 as eluent) to give 2-methyl-5-(phenyl(benzenesulfonyl)methyl) as a white solid after purification Thiophene yield was 82%. The reaction equation is show...
Embodiment 2
[0019] 4-Fluorobenzaldehyde was used to replace the benzaldehyde in Example 1 to obtain a white solid 2-((4-fluorophenyl)(benzenesulfonyl)methyl)-5-methylthiophene in a yield of 89%.
[0020] 1 H NMR (400MHz, Chloroform-d)δ7.76-7.33(m,7H),7.12-6.87(m,3H),6.63(dq,J=3.5,1.1Hz,1H),5.44(s,1H), 2.45(s,3H).
[0021] 13 C NMR (101MHz, cdcl 3 )δ164.23,161.75,142.17,137.30,133.68,131.82,131.74,130.71,129.78,129.09,128.69,128.49,125.15,115.76,115.55,71.64,15.29.
[0022] HRMS(ESI):calculated for C 18 H 15 FO 2 S 2 Na[M+Na] + =369.0395,foundC 18 H 15 FO 2 S 2 Na[M+ Na] + =369.0373.
[0023] Melting point: 129-130℃.
Embodiment 3
[0025] 2-chlorobenzaldehyde was used to replace the benzaldehyde in Example 1 to obtain a white solid 2-((2-chlorophenyl)(benzenesulfonyl)methyl)-5-methylthiophene in a yield of 88%.
[0026] 1 H NMR (400MHz, Chloroform-d) δ 8.23–8.16 (m, 1H), 7.74–7.65 (m, 2H), 7.63–7.55 (m, 1H), 7.47–7.35 (m, 3H), 7.26–7.23 (m, 2H), 7.00 (d, J=3.5Hz, 1H), 6.66 (dd, J=3.6, 1.2Hz, 1H), 6.29 (s, 1H), 2.47 (d, J=1.1Hz, 3H) .
[0027] 13 C NMR (101MHz, CDCl 3 )δ142.43,137.63,134.73,133.77,130.99,130.70,130.26,130.13,130.10,129.55,129.08,128.70,127.23,125.15,66.83,15.36.
[0028] HRMS(ESI):calculated for C 18 H 15 ClO 2 S 2 Na[M+Na] + =385.0100,foundC 18 H 15 ClO 2 S 2 Na[M+Na] + =385.0089.
[0029] Melting point: 100-101℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com