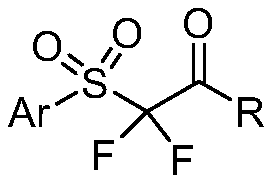
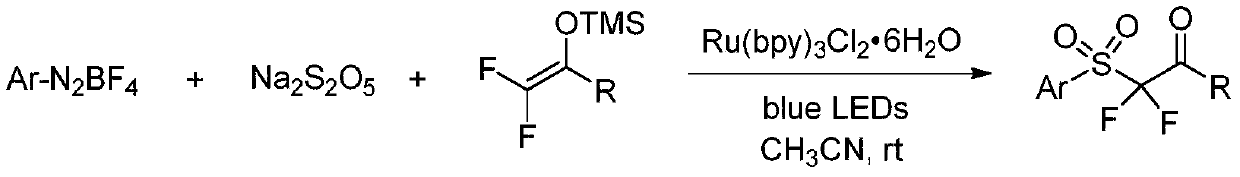
Synthesis method of alpha, alpha-difluoro-beta-carbonyl sulfone compound
A synthetic method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems that limit further applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] At room temperature, add p-tolyldiazonium salt (0.3mmol), sodium metabisulfite (0.4mmol), 2,2-difluoroenolsilyl ether (0.2mmol) and Ru(bpy) sequentially to a dry reaction tube 3 Cl 2 ·6H 2 O (0.004mmol), plugged the reaction tube with a stopper and replaced it in high-purity nitrogen for three times, so that the system was under anaerobic conditions, then added acetonitrile (2mL), and stirred under 15W blue light until the reaction was completely monitored by TLC. The reaction solution was directly concentrated under reduced pressure, and the mixed system of petroleum ether and ethyl acetate was used as the mobile phase for column chromatography to obtain the corresponding α,α-difluoro-β-carbonyl sulfone compound example 1.
[0029] Structural characterization of compound example 1: 1 H NMR (400MHz, CDCl 3)δ8.18(d, J=7.8Hz, 1H), 7.90(d, J=7.7Hz, 1H), 7.70(t, J=7.4Hz, 1H), 7.54(t, J=7.5Hz, 1H) ,7.44(d,J=7.8Hz,1H),2.50(s,2H). 13 C NMR (100MHz, CDCl 3 )δ1...
Embodiment 2
[0031]
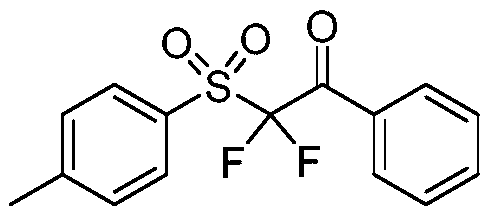
[0032] Add phenyldiazonium salt (0.3mmol), sodium metabisulfite (0.4mmol), 2,2-difluoroenol silyl ether (0.2mmol) and Ru(bpy) sequentially to a dry reaction tube at room temperature 3 Cl 2 ·6H 2 O (0.004mmol), plugged the reaction tube with a stopper and replaced it in high-purity nitrogen for three times, so that the system was under anaerobic conditions, then added acetonitrile (2mL), and stirred under 15W blue light until the reaction was completely monitored by TLC. The reaction solution was directly concentrated under reduced pressure, and the mixed system of petroleum ether and ethyl acetate was used as the mobile phase for column chromatography to obtain the corresponding α,α-difluoro-β-carbonyl sulfone compound Example 2.
[0033] Structural characterization of compound example 2: 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=7.6Hz, 1H), 8.04(d, J=7.6Hz, 1H), 7.82(t, J=7.4Hz, 1H), 7.74-7.63(m, 2H), 7.55( t,J=7.5Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ183.7(t, J=23.2...
Embodiment 3
[0035]
[0036] At room temperature, add p-chlorophenyldiazonium salt (0.3mmol), sodium metabisulfite (0.4mmol), 2,2-difluoroenol silyl ether (0.2mmol) and Ru(bpy) sequentially to the dry reaction tube 3 Cl 2 ·6H 2 O (0.004mmol), plugged the reaction tube with a stopper and replaced it in high-purity nitrogen for three times, so that the system was under anaerobic conditions, then added acetonitrile (2mL), and stirred under 15W blue light until the reaction was completely monitored by TLC. The reaction liquid was directly concentrated under reduced pressure, and the mixed system of petroleum ether and ethyl acetate was used as the mobile phase for column chromatography to obtain the corresponding α,α-difluoro-β-carbonyl sulfone compound example 3.
[0037] Structural characterization of compound example 3: 1 H NMR (400MHz, CDCl 3 )δ8.17(d, J=7.8Hz, 1H), 7.97(d, J=8.0Hz, 1H), 7.72(t, J=7.4Hz, 1H), 7.64(d, J=7.9Hz, 1H) ,7.56(t,J=7.6Hz,1H). 13 CNMR (100MHz, CDCl 3 )δ183....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com