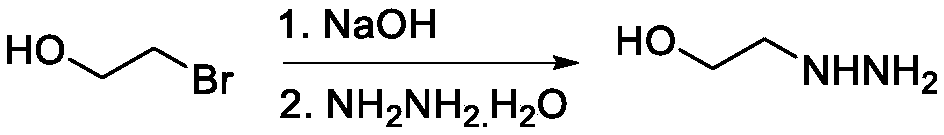Synthetic method of furazolidone metabolite AOZ
A technology of oxazolone and reaction, which is applied in the field of chemical synthesis, can solve the problems of poor recovery rate and low synthesis yield, and achieve the effect of reducing sample loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
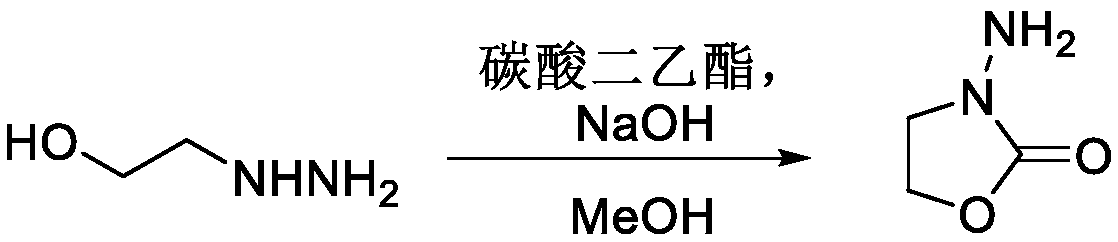
[0022] Embodiment 1, synthetic 3-amino-2-oxazolone (AOZ)
[0023]
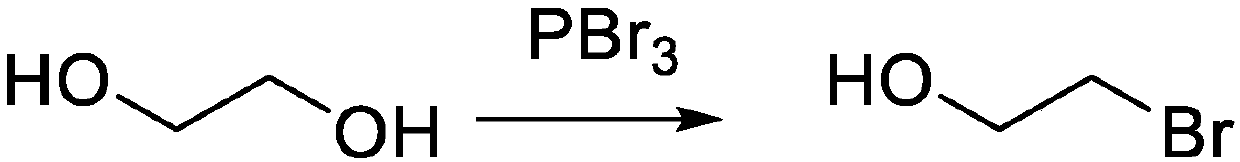
[0024] Step 1: Place ethylene glycol (5g, 1.0 equivalents) in an ice-water bath, add PBr dropwise 3 (2.6 mL, 0.377 equivalents) for 5 min, then warmed to room temperature, and finally heated to the reflux temperature of ethylene glycol, and reacted for 3 hours. After the reaction was completed, the solvent was distilled to obtain the reaction intermediate product 2-bromoethanol (6.0 g, yield 60%). For the reaction flow chart, see figure 1 .
[0025] Step 2: first dissolve the reaction product 2-bromoethanol (1.0 equivalent, 6.0g) in water, add NaOH (1.1 equivalent, 0.512g), react at 100°C for 3h, then cool down to 70°C and add hydrazine hydrate (3.0 Equivalent, 1.75 g) (after diluting hydrazine hydrate with 5 mL of water) reacted overnight. After the reaction was complete, excess 2-bromoethanol and water were removed by vacuum distillation to obtain intermediate 2-hydrazinoethanol (2.6 g, yield 70%). F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com