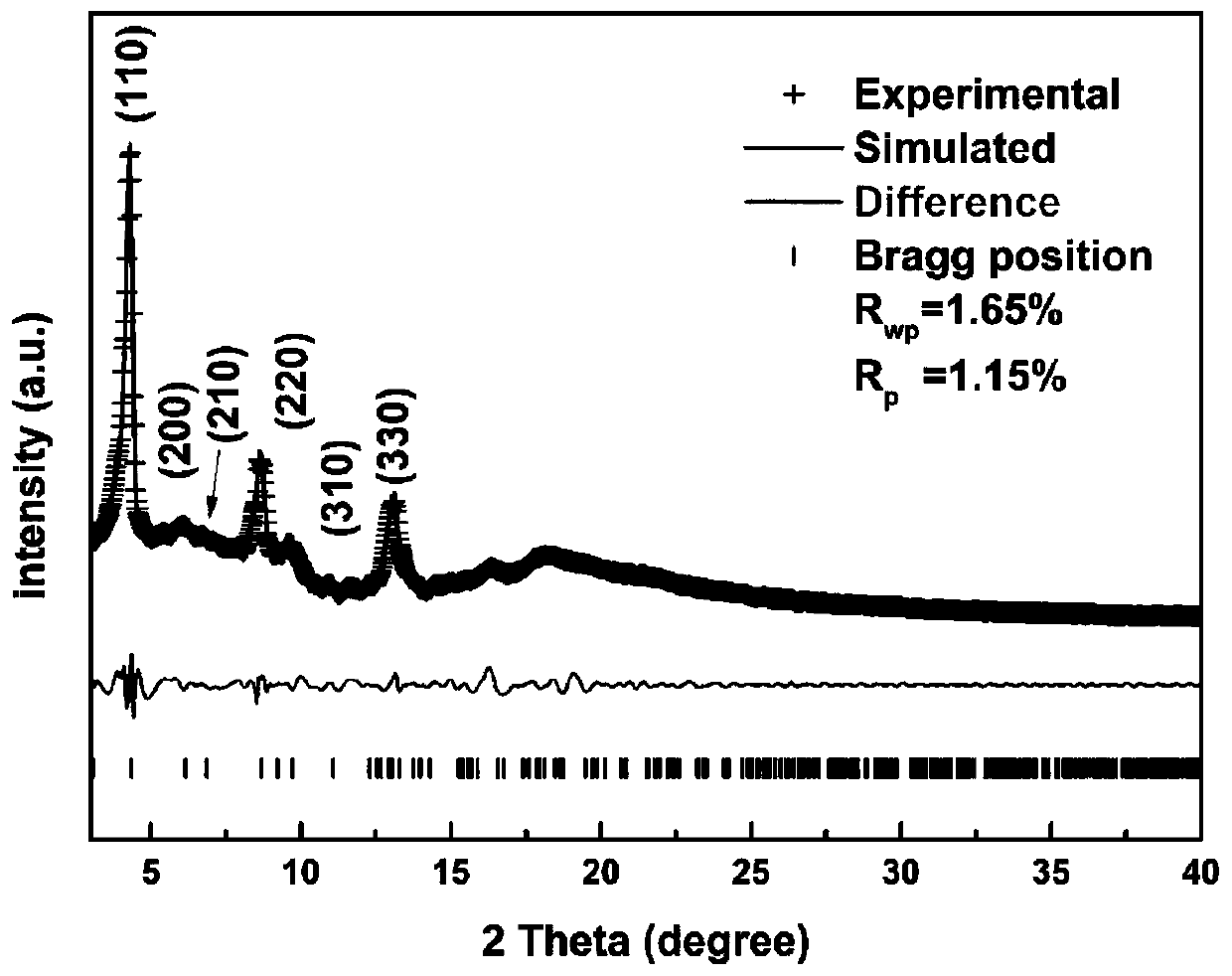
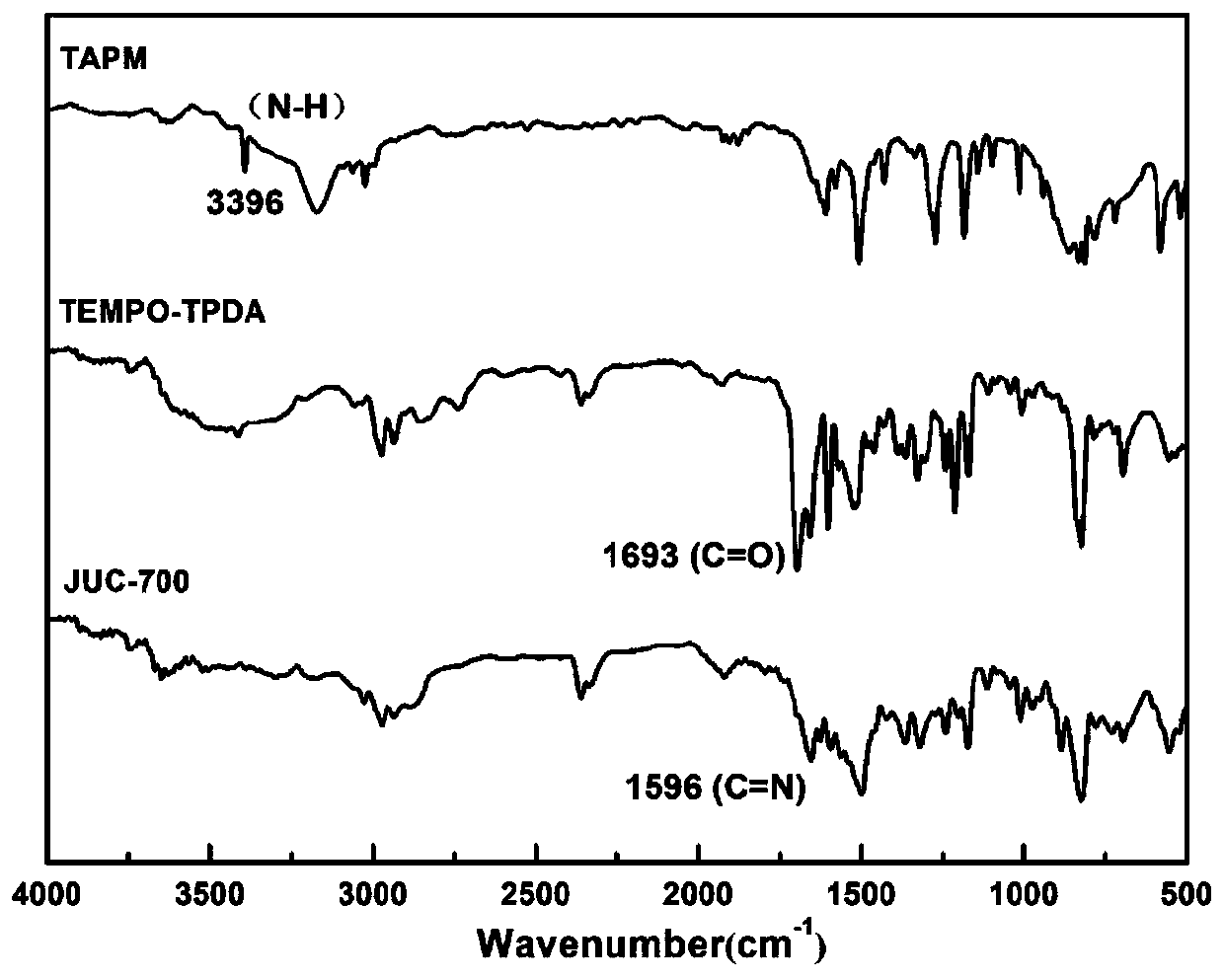
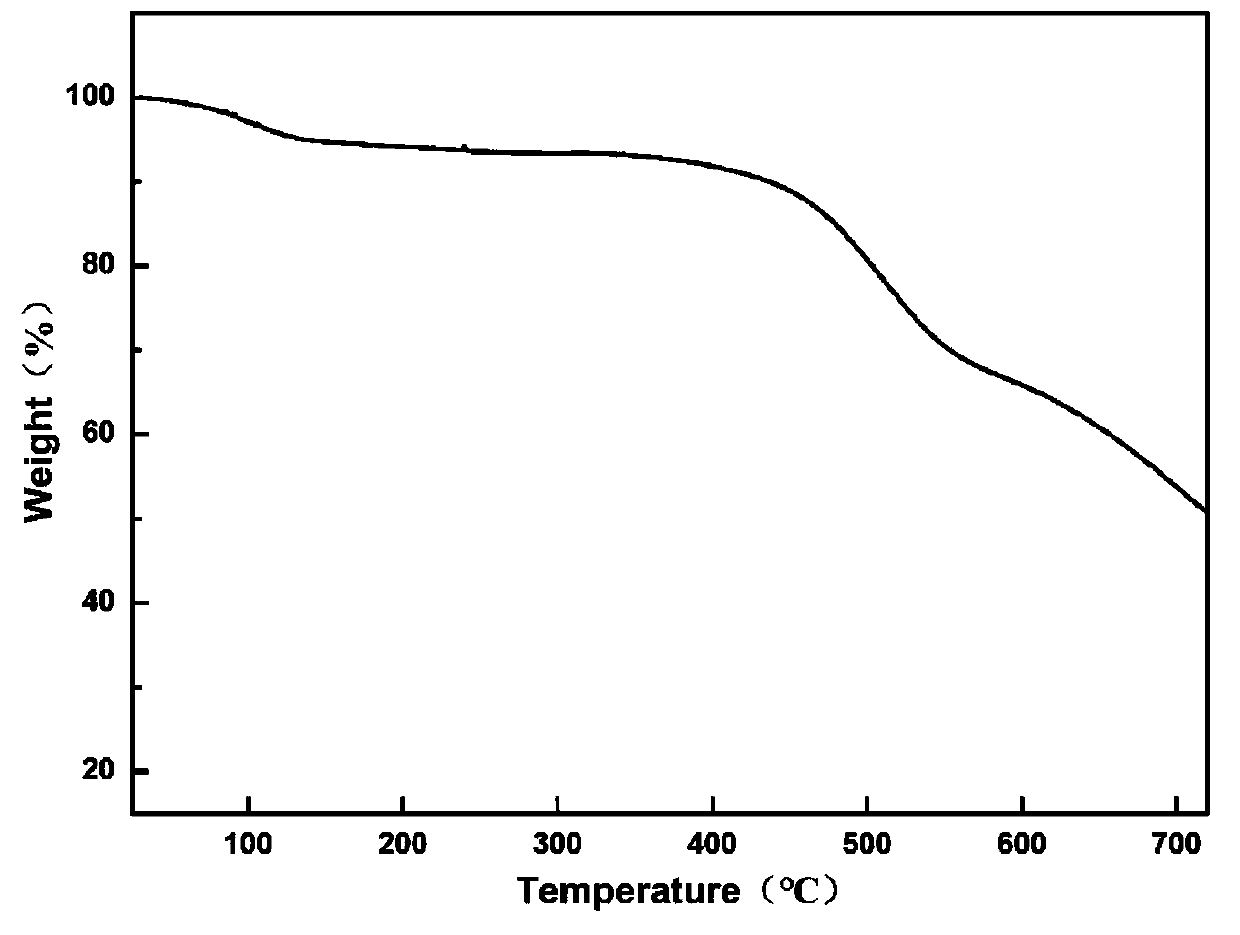
Nitroxide free radical functionalized three-dimensional covalent organic framework material and preparation method thereof
A technology of covalent organic framework and nitroxide free radicals, which is applied in the field of synthesis of three-dimensional covalent organic framework materials, can solve problems such as difficulties in the synthesis of three-dimensional covalent organic framework materials, and achieve the effect of avoiding damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A three-dimensional covalent organic framework material functionalized with nitroxide free radicals (TEMPO), which is composed of 4,7-bis(4-formylphenyl)-2,2,6,6-tetramethylpiperidine-N -Oxygen free radicals and tetrakis (4-aminophenyl) methane form the organic framework structure by Schiff base reaction, and its structural formula is as follows:
[0037]
[0038] Further, the chemical structural formula of the 4,7-bis(4-formylphenyl)-2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO-TPDA) is as follows :
[0039]
[0040] A preparation method of a three-dimensional covalent organic framework material functionalized by nitroxide free radicals (TEMPO), the specific steps are as follows:
[0041] (1) Synthesis of 4,7-bis(4-bromophenyl)-2,2,6,6-tetramethylpiperidine-N-oxyl radical:
[0042]
[0043] Dissolve 2,5-dibromobenzoic acid (8.06g) in 32mL of thionyl chloride, reflux at 80°C for 3h, cool to room temperature after the reaction, and spin dry to remove th...
Embodiment 2
[0055] Step (1), (2) are with embodiment 1,
[0056] (3) Tetrakis(4-phenylamino)methane (TAPM, (15mg) and 4,7-bis(4-formylphenyl)-2,2,6,6-tetramethylpiperidine-N-oxygen Free radicals (TEMPO-TPDA, 35.5mg) were thoroughly mixed in a mortar and then added to a glass tube, then slowly added 0.5mL 1,2-o-dichlorobenzene, 0.75mL 1-butanol, 0.1ml acetic acid (3mol / L), inflated three times under the nitrogen atmosphere, then the glass tube is placed in the freezing reaction system in liquid nitrogen, and then the glass tube is blocked under the flame of methane / oxygen. The prepared glass tube reaction system is placed in 120 ℃ oven for 5 days. After the reaction is over, open the glass tube with a glass cutter, wash the product with N,N-dimethylformamide and acetone three times, and then filter it. The solid product is vacuum-dried at 80°C After drying in the oven for 2 hours, a tan target product was obtained with a yield of 80%. Subsequent characterization was carried out after soa...
Embodiment 3
[0058] Tetrakis(4-anilino)methane (TAPM, (15 mg) and 4,7-bis(4-formylphenyl)-2,2,6,6-tetramethylpiperidine-N-oxyl radical ( TEMPO-TPDA, 35.5mg) was mixed thoroughly in a mortar and then added to a glass tube, then slowly added 0.5mL 1,2-o-dichlorobenzene, 0.75mL 1-butanol, 0.1ml acetic acid (4mol / L) , pump and inflate three times under a nitrogen atmosphere, then place the glass tube in liquid nitrogen to freeze the reaction system, and then seal the glass tube under a methane / oxygen flame. Place the prepared glass tube reaction system in an oven at 140°C 3 days. After the reaction is over, open the glass tube with a glass cutter, wash the product three times with N,N-dimethylformamide and acetone, and then filter. Dry the solid product in a vacuum oven at 80°C After 2 hours, the brown-colored target product was obtained with a yield of 79%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap