Storage method of acetoin
A technology of acetoin and acetic acid, which is applied in the field of storage that effectively reduces the decomposition of acetoin, can solve the problems of decreased purity of acetoin, short shelf life, and accelerated rapid decomposition of acetoin monomers, etc., to achieve the effect of improving storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
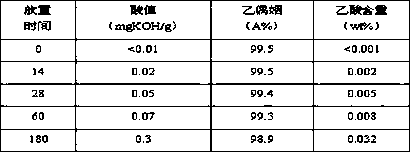
[0040] (1) Place the acetoin monomer Z prepared by the dehydrogenation method under light conditions for 10 days, and measure the mass content of acetic acid to be 0.28% to obtain acetoin monomer A-1; (2) make A -1 at 0.1MPa, 85°C, liquid space velocity 0.1 h -1Under the same conditions, the acetoin monomer B-1 was obtained through an adsorption exchange bed filled with Ca / FER molecular sieves, and the Ca of B-1 was measured by ICP 2+ The content was 117 μg / g; (3) Seal B-1 in a narrow bottle to obtain a sample of C-1, and store it at room temperature. The changes of various indicators of the C-1 sample with the storage time were measured, and the results are listed in Table 2.
[0041] surface
[0042]
Embodiment 2
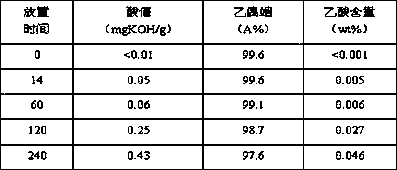
[0044] (1) Add an appropriate amount of analytically pure acetic acid to the acetoin monomer Z prepared by the dehydrogenation method to prepare acetoin monomer A-2 with a mass content of 0.12% acetic acid; (2) make A-2 at 0.5 MPa, 50℃, liquid space velocity 0.01 h -1 Under the same conditions, the acetoin monomer B-2 was obtained through an adsorption exchange bed filled with Ca / FER molecular sieves, and the Ca of B-2 was measured by ICP 2+ The content was 163 μg / g; (3) The sample of C-2 was obtained by sealing B-2 in a narrow bottle and stored at room temperature. The changes of various indicators of the C-2 sample with the storage time were measured, and the results are listed in Table 3.
[0045] table 3
[0046]
Embodiment 3
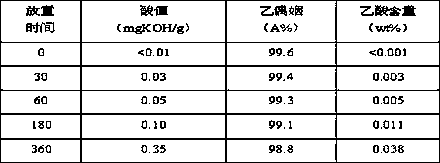
[0048] (1) Place the acetoin monomer Z prepared by the dehydrogenation method under light conditions for 5 days, and measure the mass content of acetic acid to be 0.21%, to obtain the acetoin monomer A-3; (2) make A -3 at 0.1MPa, 75°C, liquid space velocity 0.05 h -1 Under certain conditions, the acetoin monomer B-3 was obtained through an adsorption exchange bed filled with Ca / FER molecular sieves, and the Ca of B-3 was measured by ICP 2+ The content was 193 μg / g; (3) Seal B-3 in a narrow bottle to obtain a sample of C-3, and store it at room temperature. The changes of various indicators of the C-3 sample with the storage time were measured, and the results are listed in Table 4.
[0049] Table 4
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com