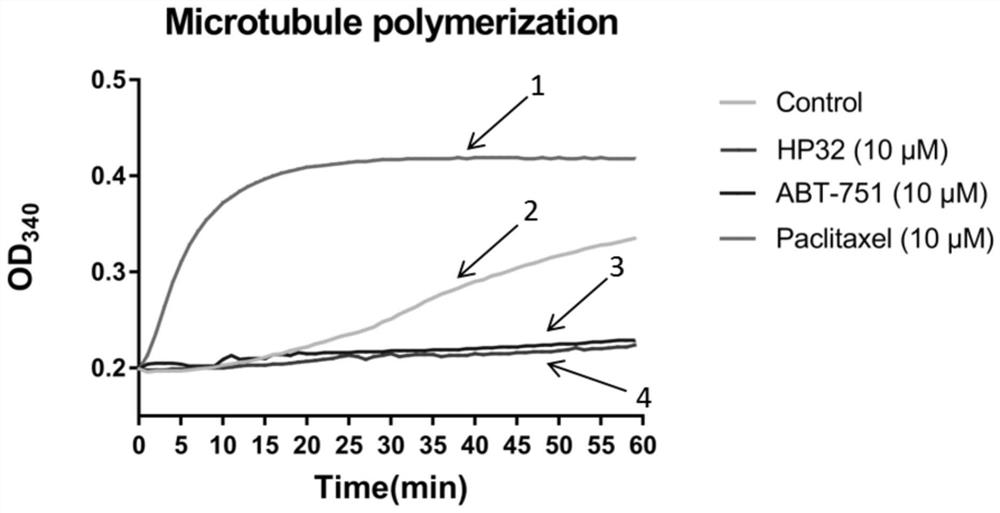
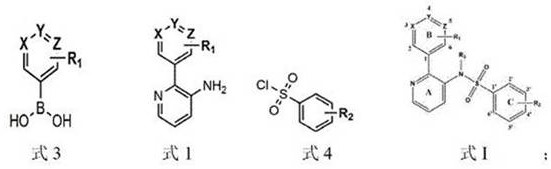
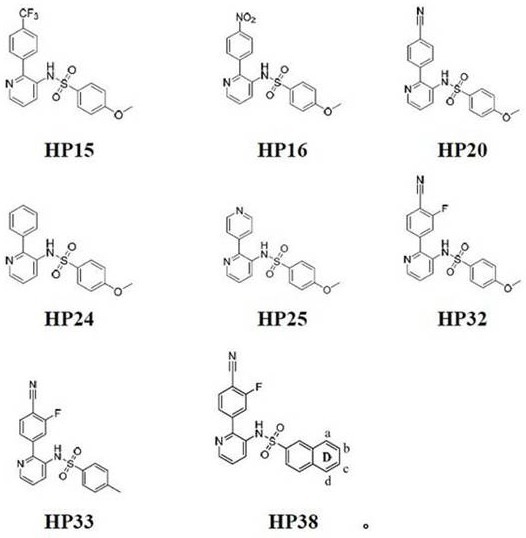
Pyridine-2-aryl-3-sulfonamide compound and its synthesis method and application
A technology of sulfonamides and synthesis methods, applied in the preparation of anti-tumor drugs or tubulin polymerization inhibitors, pyridine-2-aryl-3-sulfonamides and their synthesis fields, can solve the problem of high structural toxicity, Toxic side effects, drug resistance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 representative compound HP1:
[0037] 1. Synthesis of intermediate 2-(2-methoxyphenyl)pyridine-3-amino (step a): Weigh 2-bromo-3-aminopyridine (1,500 mg) and 2-methoxyphenylboronic acid ( 483mg, 1.1eq) was dissolved in toluene / water mixed solvent (2 / 1, v / v), potassium carbonate (1.6g, 4eq) and Pd(PPh 3 ) 4 (167mg, 0.05eq) was used as a catalyst, and the reaction bottle was stirred and reacted for 8 hours at 80°C under a nitrogen atmosphere, and then the reaction was terminated. The solvent was distilled off under reduced pressure, extracted 3 times with ethyl acetate / water, the ethyl acetate layer was taken, spin-dried, and purified by silica gel column chromatography to obtain the intermediate 2-(2-methoxyphenyl)pyridine-3- Amino group (310 mg, 60% yield).
[0038]2. Synthesis of the final product HP1 (step b): Weigh and dissolve the intermediate 2-(2-methoxyphenyl)pyridine-3-amino (100mg) and 4-dimethylaminopyridine (DMAP, 61mg, 1eq)...
Embodiment 2
[0040] The preparation of embodiment 2 representative compound HP2:
[0041] 1. The synthesis of intermediate 2-(3-methoxyphenyl)pyridine-3-amino: the reaction conditions and post-treatment method are the same as the preparation step a of the representative compound HP1, the difference is that the reaction raw material 2-methoxy Phenylboronic acid was replaced by 3-methoxyphenylboronic acid, and the yield was 70%.
[0042] 2. Synthesis of the final product HP2: the reaction conditions and post-treatment method are the same as the preparation step b of the representative compound HP1, the difference is that the reaction raw material intermediate 2-(2-methoxyphenyl)pyridine-3-amino is replaced by 2-(3-Methoxyphenyl)pyridin-3-amino, 65% yield.
[0043] Compound (HP2): 4-methoxy-N-(2-(3-methoxyphenyl)pyridin-3-yl)benzenesulfonamide. white solid. Melting point: 132.3-133.1°C. 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 9.73 (1H, s), 8.48 (1H, dd), 7.54 (1H, dd), 7.47 (2H, d), 7.34-7.2...
Embodiment 3
[0044] The preparation of embodiment 3 representative compound HP3:
[0045] 1. The synthesis of intermediate 2-(4-methoxyphenyl)pyridine-3-amino: the reaction conditions and post-treatment method are the same as the preparation step a of the representative compound HP1, the difference is that the reaction raw material 2-methoxy Phenylboronic acid was replaced by 4-methoxyphenylboronic acid, yield 75%.
[0046] 2. Synthesis of the final product HP3: the reaction conditions and post-treatment method are the same as the preparation step b of the representative compound HP1, the difference is that the reaction raw material intermediate 2-(2-methoxyphenyl)pyridine-3-amino is replaced by 2-(4-Methoxyphenyl)pyridin-3-amino, 60% yield.
[0047] Compound (HP3): 4-methoxy-N-(2-(4-methoxyphenyl)pyridin-3-yl)benzenesulfonamide. Pale yellow solid. Melting point: 166.8-167.7°C. 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.38(1H,dd),8.01(1H,dd),7.53(2H,d),7.23(1H,dd),7.04(2H,d),6.91(2H,d),6.86( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com