Method for preparing oxaziclomefone
A technology of oxaziclozone and its compound, which is applied in the field of preparing oxaziclomem, can solve the problems of complex process route, low product purity, and unsuitability for large-scale industrial production, and achieve the effect of simple process steps and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
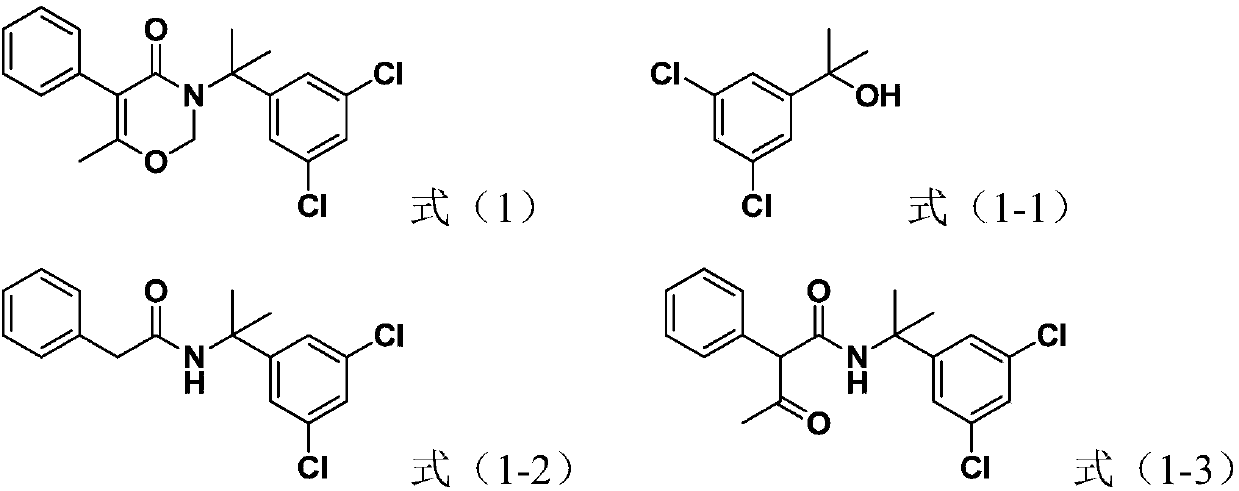
[0046] Oxaziclozone having the structure represented by formula (1) was prepared.
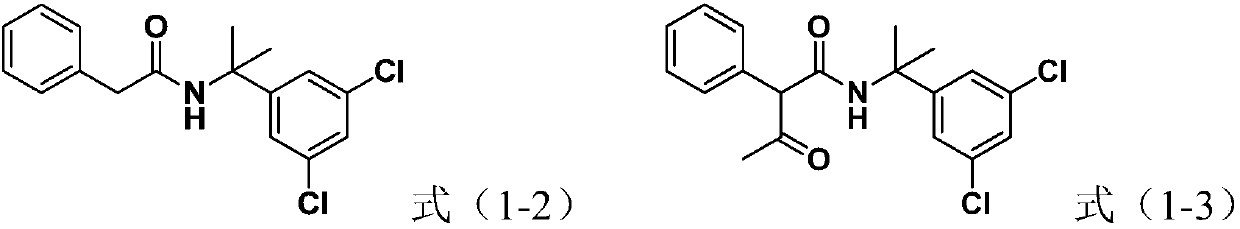
[0047] 1) At 0°C, add the compound (0.1mol), phenylacetonitrile (0.11mol) and toluene (100mL) represented by formula (1-1) into the reaction flask, stir well, add concentrated sulfuric acid (98 wt. %, the same below) total 20g, and control the speed of dripping the concentrated sulfuric acid so that the liquid temperature in the reaction bottle is not higher than 10°C, after the dropwise addition of the concentrated sulfuric acid, continue to react for 8h (start timing from the beginning of the dripping of the concentrated sulfuric acid), and then Add 100 mL of water to the reaction system, add aqueous sodium carbonate solution dropwise until the pH of the solution is 7, filter, and dry to obtain the compound shown in formula (1-2), with a yield of 94.4% and a purity of 93.9%;
[0048]2) At 0°C, sodium methoxide (0.2mol) and methyl acetate (2.5mol) were added to the reaction flask, stirred even...
Embodiment 2
[0051] Oxaziclozone having the structure represented by formula (1) was prepared.
[0052] 1) At -4°C, add the compound (0.1mol), phenylacetonitrile (0.12mol) and toluene (100mL) represented by formula (1-1) into the reaction flask, stir well, add concentrated sulfuric acid (98 % by weight, the same below) is 18g in total, and the speed of dripping the vitriol oil is controlled so that the liquid temperature in the reaction bottle is not higher than 10°C, and after the vitriol dripping, continue to react for 12h (starting to count from the vitriol dripping), Then add 100mL water to the reaction system, add dropwise sodium carbonate aqueous solution until the pH value of the solution is 7, filter and dry to obtain the compound shown in formula (1-2), with a yield of 96.5% and a purity of 96.2%;
[0053] 2) At -10°C, add sodium ethoxide (0.3mol) and ethyl acetate (2.5mol) into the reaction flask, stir evenly, and add dropwise the ethyl acetate solution containing the compound re...
Embodiment 3
[0056] The present embodiment adopts the method similar to embodiment 1 to carry out, and the difference is, in the step 2) of the present embodiment, control the speed of dripping the methyl acetate solution containing the compound shown in formula (1-2) so that the reaction The liquid temperature in the bottle is not higher than 20°C.
[0057] As a result, the yield of the compound shown in formula (1-3) was 85.1%, and the purity was 97.2%;
[0058] The same method as in Example 1 was used to prepare oxaziclozone represented by formula (1) from the compound represented by formula (1-3) in this example, with a yield of 74% and a purity of 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com