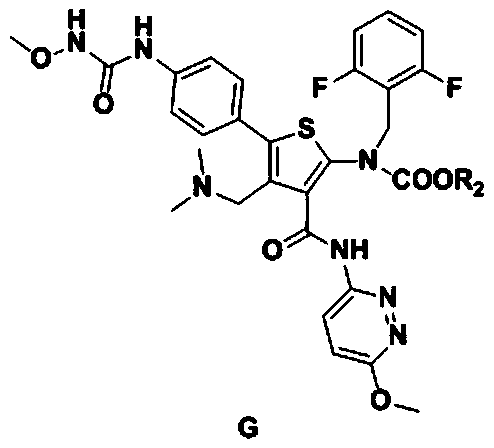
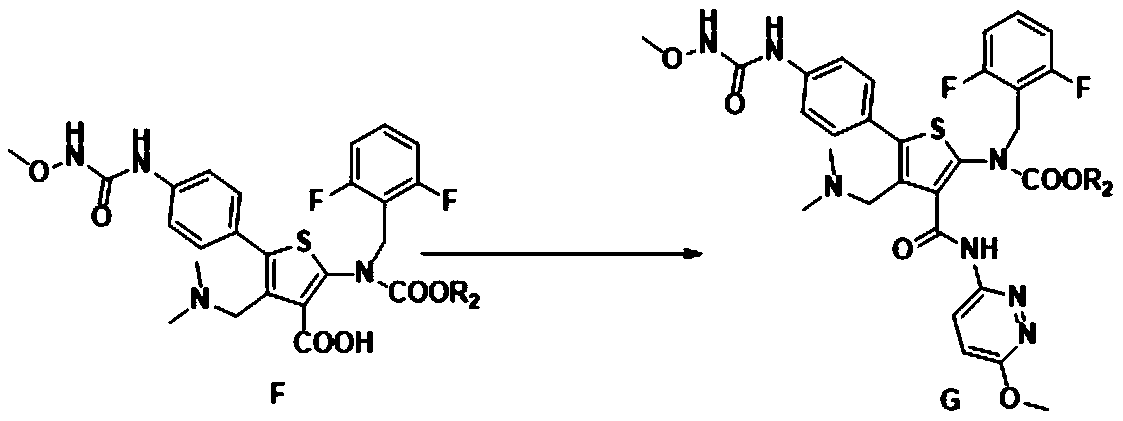
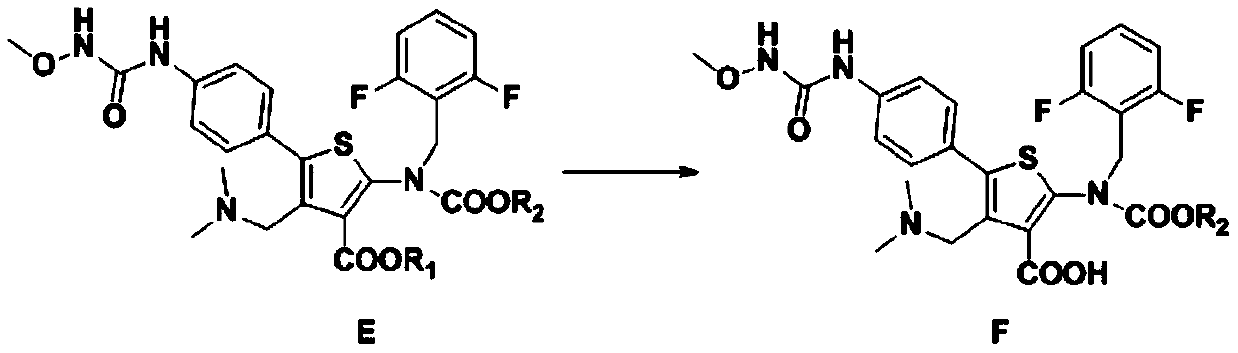
Relugolix intermediate compound, preparation method and application thereof
A compound and action technology, applied in the field of chemical drug synthesis, can solve the problems of increased removal difficulty, less urea by-products, and increased impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0089] Embodiment 1-2: formula B (wherein R 1 is ethyl, R 2 is isobutyl, X is the preparation of intermediate shown in bromine)
[0090]
Embodiment 1
[0092] Weigh 30.14g of compound formula A and add it to a 1000mL three-necked flask, then add 240mL of ethyl acetate, 15.11g of NBS (N-bromosuccinimide) and 0.93g of AIBN (azobisisobutyronitrile), and stir to 70°C, reacted for 18 hours and then lowered to room temperature, added 60mL saturated sodium sulfite solution, then added 200mL water, stirred for 10min, separated the water phase, and washed the organic phase twice with 100mL water. Concentrate the organic phase to about 120 mL under reduced pressure, add 90 mL of ethanol, and concentrate the mixture to about 120 mL, then add 90 mL of ethanol, and then concentrate to 120 mL, add 50 mL of heptane, and stir the mixture at 20 to 30 ° C for 30 Minutes, add n-heptane again, stir at 0 to 10°C for 1 hour, collect the crystals by filtration, wash with ethanol / heptane=1 / 2, and dry under reduced pressure to obtain 31.64g light yellow crystal formula B, yield 91.45%, The chemical purity is 97.80%.
[0093] The NMR data are as foll...
Embodiment 2
[0095] Weigh 30.14g of compound formula A and add it to a 1000mL three-necked flask, then add 240mL of chloroform, 15.11g of NBS (N-bromosuccinimide) and 0.93g of AIBN (azobisisobutyronitrile), and stir to heat up to 60 ℃, reacted for 15 hours and lowered to room temperature, added 60mL of saturated sodium sulfite solution, then added 200mL of water, stirred for 10min and separated the water phase, and the organic phase was washed twice with 100mL of water. Concentrate the organic phase to about 120 mL under reduced pressure, add 90 mL of ethanol, and concentrate the mixture to about 120 mL, then add 90 mL of ethanol, and then concentrate to 120 mL, add 50 mL of heptane, and stir the mixture at 20 to 30 ° C for 30 Minutes, add n-heptane again, stir at 0 to 10°C for 1 hour, collect the crystals by filtration, wash with ethanol / heptane=1 / 2, and dry under reduced pressure to obtain 31.95g light yellow crystal formula B, yield 92.33%, The chemical purity is 97.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com