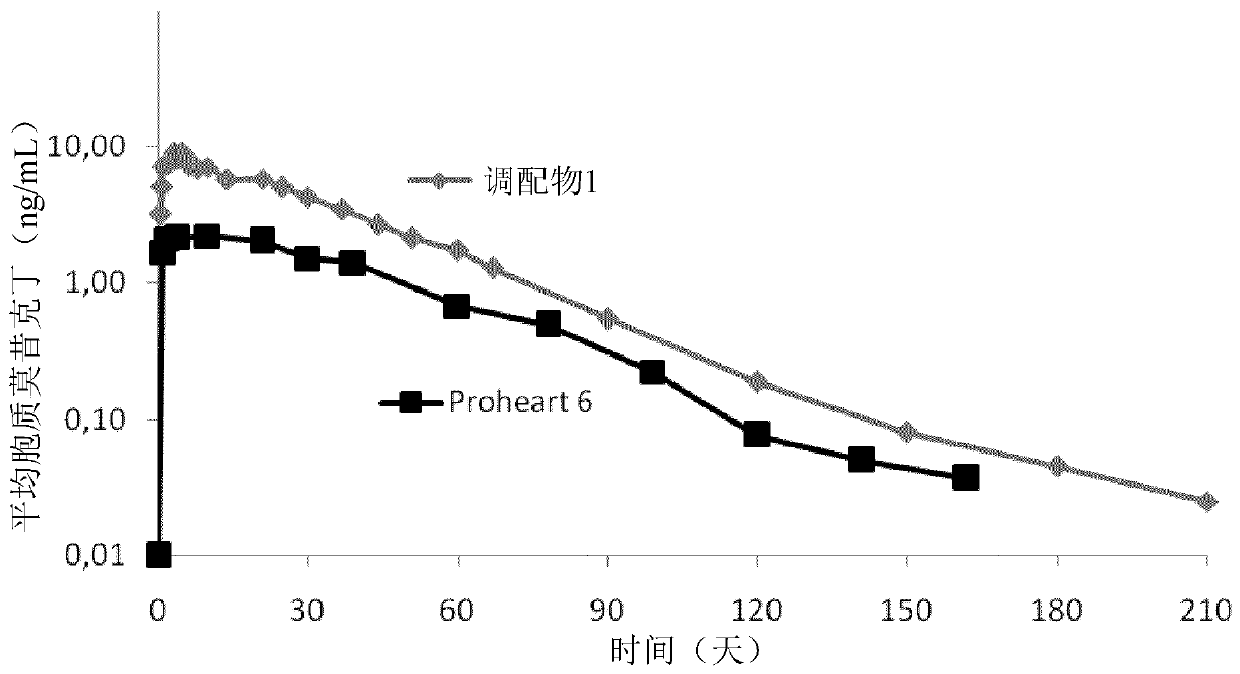
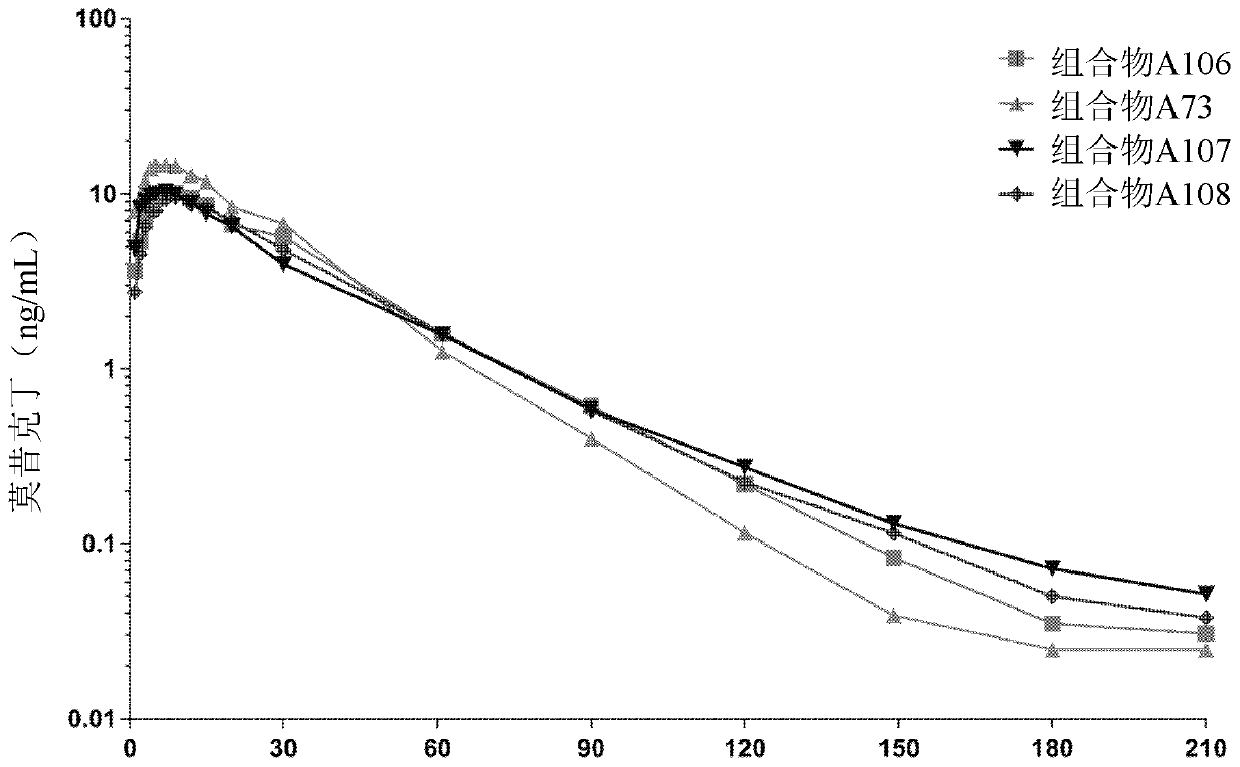
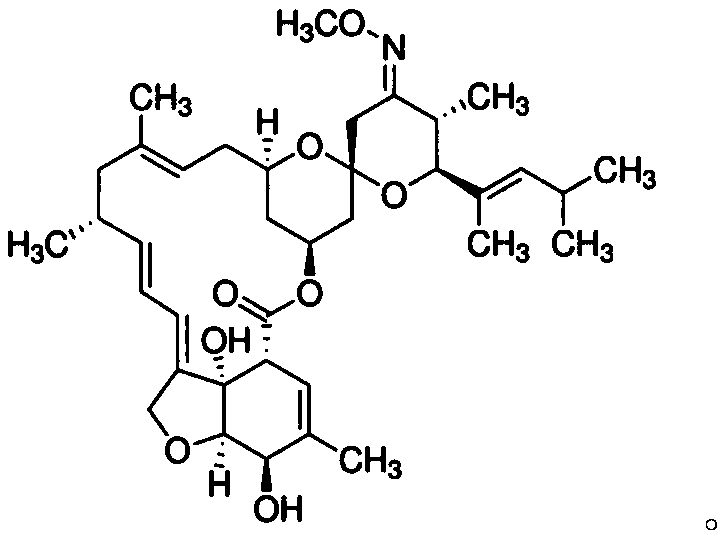
Composition containing moxidectin for treating parasites infestations
A technology of moxidectin and composition, applied in the field of treating and/or preventing parasitic infection of non-human mammals, can solve the problems of local tolerance, undisclosed, complicated preparation and use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0183] Example 1: Formulation 1
[0184] In a manufacturing vessel, a moxidectin solution according to the composition depicted below has been prepared. With stirring a portion of benzyl alcohol, butylated hydroxytoluene (BHT), propylene carbonate and moxidectin have been added. Then, the volume was replenished with benzyl alcohol.
[0185]
example 2
[0186] Example 2: Formulation 2
[0187] In a manufacturing vessel, a moxidectin solution according to the composition depicted below has been prepared. With stirring a portion of isopropanol, BHT, dipropylene glycol monomethyl ether (DPGME) and moxidectin have been added. Then, make up the volume with isopropanol.
[0188]
example 3
[0189] Example 3: Formulation 3
[0190] The same method as Example 1 has been used.
[0191]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com