COVID-19 rapid diagnosis kit and preparation method thereof
A COVID-19, rapid diagnosis technology, applied in the field of disease detection, can solve the problem of no specific treatment for the disease, and achieve the effect of being conducive to effective control and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of COVID-19 rapid diagnostic kit, comprises colloidal gold of colloidal gold of colloidal protein of COVID-19 fibril protein, is prepared by following method:
[0032] 1. Expression and purification of COVID-19 spike protein
[0033] 1) Fully synthesize the gene encoding the Gln14-Arg685 region of the S1 protein;
[0034] 2) Cloning the S1 gene between the IL2 secretion signal peptide of the eukaryotic expression vector pMFcIg (ABLINK biotech) and the mouse Fc (mFc, including hinge-CH2-CH3) tag gene to form the S1-mFc fusion gene, the cloning method used The forward primer is: CAGTGTGTTAATCTTACAACC, the reverse primer is: ACGTGCCCGCCGAGGAGAATT;
[0035] 3) Transfer the S1-mFc expression plasmid to the competent cell Rosseta by electroporation, pick the positive single clone, and extract the plasmid after amplification;
[0036] 4) Use 293Fectin (thermofisher) transfection reagent to transfect S1-mFc expression plasmid into 293F cells, continue to culture in ser...
Embodiment 2
[0045] Clinical verification of colloidal gold for COVID-19 spike protein antigen prepared by the method in Example 1.
[0046] Take 8 clinically confirmed cases, 10 suspected samples and 10 negative samples of new coronavirus pneumonia for verification. The verification method is as follows:
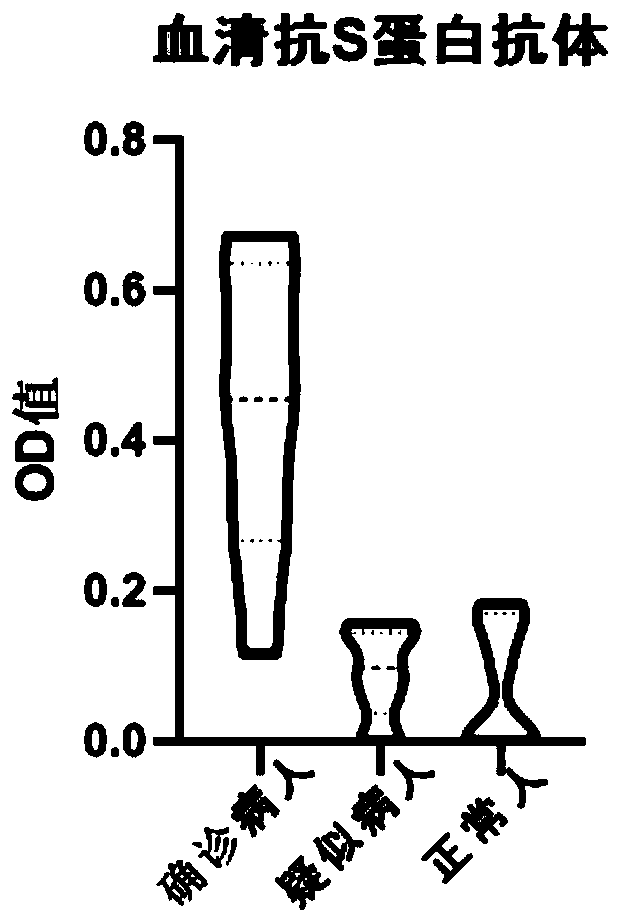
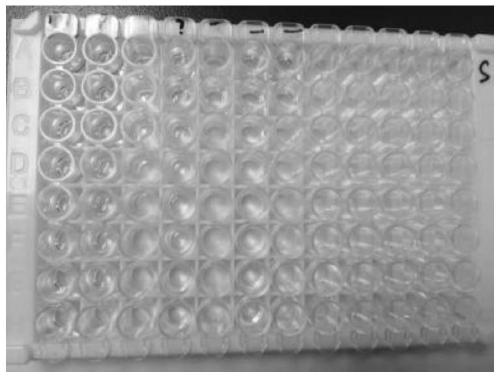
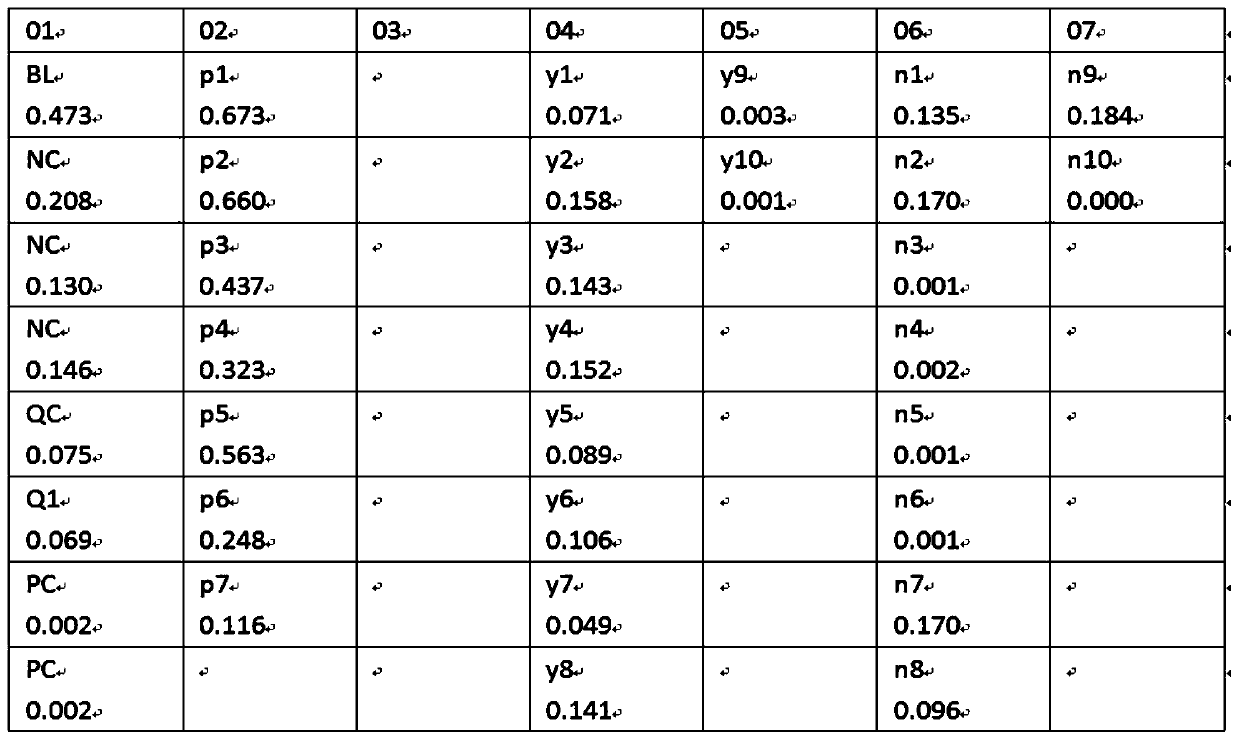
[0047] Add COVID-19 spike protein antigen colloidal gold into the 96-well plate, add 100ul of the above-mentioned different types of specimens respectively, incubate at 37 degrees for 1 hour, wash the plate, then add the enzyme-labeled secondary antibody against the Fc segment of human IgG, and develop the color reagent for color development, and the color development results are as follows figure 1, using a microplate reader to measure the OD value, the results are as follows figure 2 , 3 shown.
[0048] Clinically confirmed positive (8 cases), suspected samples (10 cases) and negative samples (10 cases) were analyzed. The serum anti-S protein antibodies of positive patients were a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com