Preparation method and use method of Li4Mn5O12 nanosheet material
A nanosheet and electrodeposition technology, which is applied in the field of preparation of Li4Mn5O12 nanosheet materials, can solve the problems of poor electrochemical performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
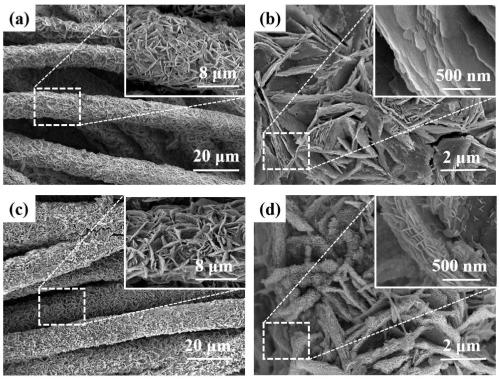
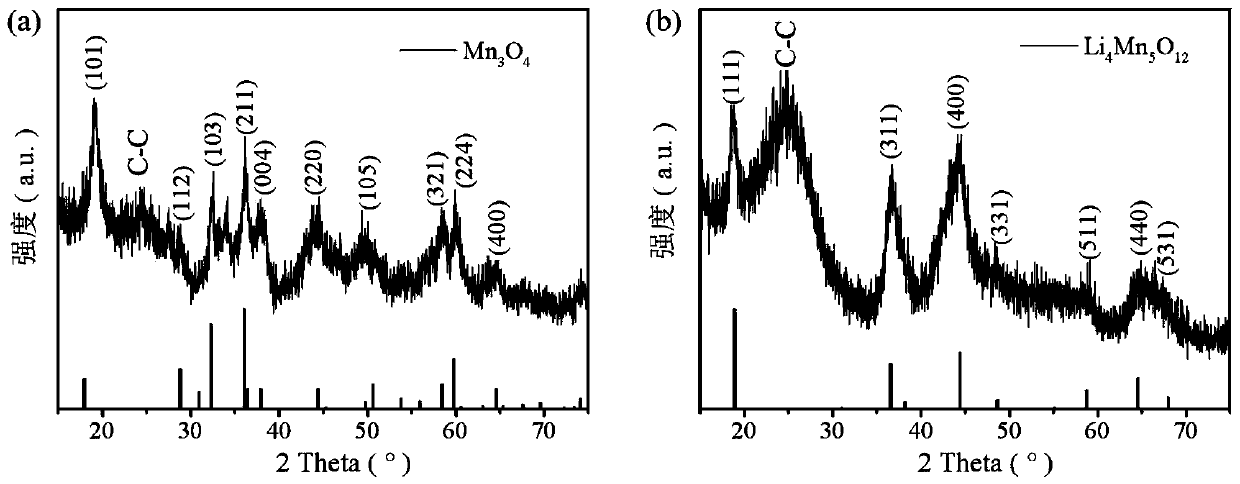
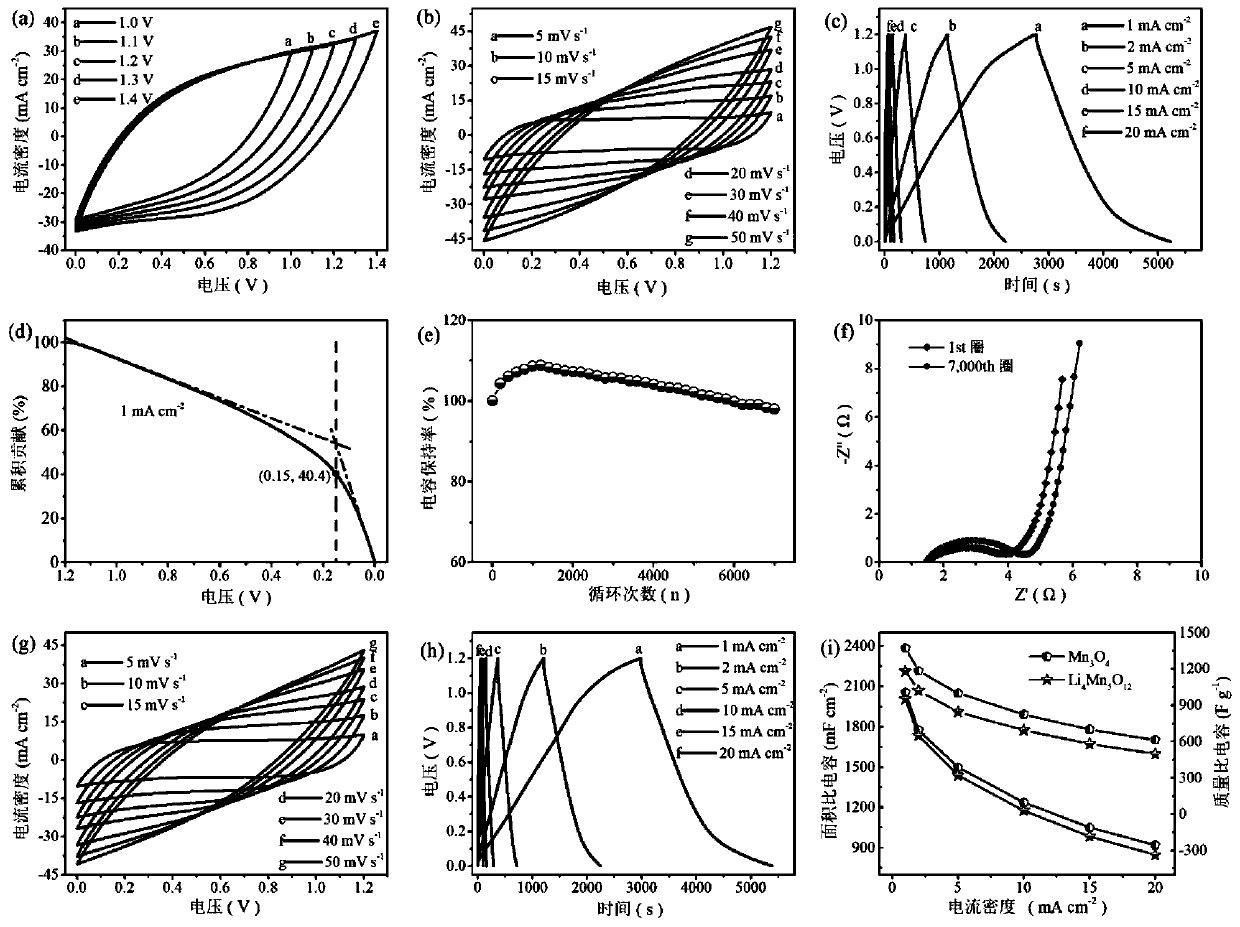
[0028] Step 1, 10mmol Mn(CH 3 COO) 2 4H 2 O and 10 mmol Na 2 SO 4 Dissolve in 100mL deionized water to form the first mixed solution; use the first mixed solution as the first electrolyte, the carbon cloth as the working electrode, the Pt sheet as the counter electrode and the Ag / AgCl electrode as the reference electrode in a three-electrode system Under the constant voltage mode, the reaction was carried out in the constant voltage mode, the voltage was set to -2.0V, and the reaction time was 800s; after the reaction, Mn(OH) was generated on the carbon cloth 2 Nanosheet precursors were washed three times with deionized water and ethanol solvent, and dried in a vacuum oven at 50 °C for 5 hours, Mn(OH) 2 Nanosheet precursors can be converted into Mn 3 o 4 Nanosheets;
[0029] Step two, 150mmol Li 2 SO 4 Dissolve in 100mL deionized water to form a uniform second solution; using the second solution as the second electrolyte, there will be Mn 3 o 4 The carbon cloth of t...
Embodiment 2
[0032] Step 1, 12mmol Mn(CH 3 COO) 2 4H 2 O and 12 mmol Na 2 SO 4 Dissolve in 100mL deionized water to form the first mixed solution; use the first mixed solution as the first electrolyte, the carbon cloth as the working electrode, the Pt sheet as the counter electrode and the Ag / AgCl electrode as the reference electrode in a three-electrode system Under the constant voltage mode, the reaction was carried out in the constant voltage mode, the voltage was set to -1.9V, and the reaction time was 900s; after the reaction, Mn(OH) was generated on the carbon cloth 2 Nanosheet precursors were washed three times with deionized water and ethanol solvent, and dried in a vacuum oven at 60 °C for 5 hours, Mn(OH) 2 Nanosheet precursors can be converted into Mn 3 o 4 Nanosheets;
[0033] Step 2, 250mmol Li 2 SO 4 Dissolve in 100mL deionized water to form a uniform second solution; using the second solution as the second electrolyte, there will be Mn 3 o 4 The carbon cloth of the...
Embodiment 3
[0036] Step 1, 8mmol Mn(CH 3 COO) 2 4H 2 O and 10 mmol Na 2 SO 4 Dissolve in 100mL deionized water to form the first mixed solution; use the first mixed solution as the first electrolyte, the carbon cloth as the working electrode, the Pt sheet as the counter electrode and the Ag / AgCl electrode as the reference electrode in a three-electrode system Under the constant voltage mode, the reaction was carried out in the constant voltage mode, the voltage was set to -1.8V, and the reaction time was 1200s; after the reaction, Mn(OH) was generated on the carbon cloth 2 Nanosheet precursors were washed three times with deionized water and ethanol solvent, and dried in a vacuum oven at 60 °C for 6 hours, Mn(OH) 2 Nanosheet precursors can be converted into Mn 3 o 4 Nanosheets;
[0037] Step two, with 200mmol Li 2 SO 4 Dissolve in 100mL deionized water to form a uniform second solution; using the second solution as the second electrolyte, there will be Mn 3 o 4 The carbon cloth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com