Method for gold recovery and extraction from electronic waste or gold containing minerals, ores and sands
A technology for recovering gold and metals, applied in the field of gold recovery, which can solve the problems of unsustainable environment, unattractive economy, unfavorable environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
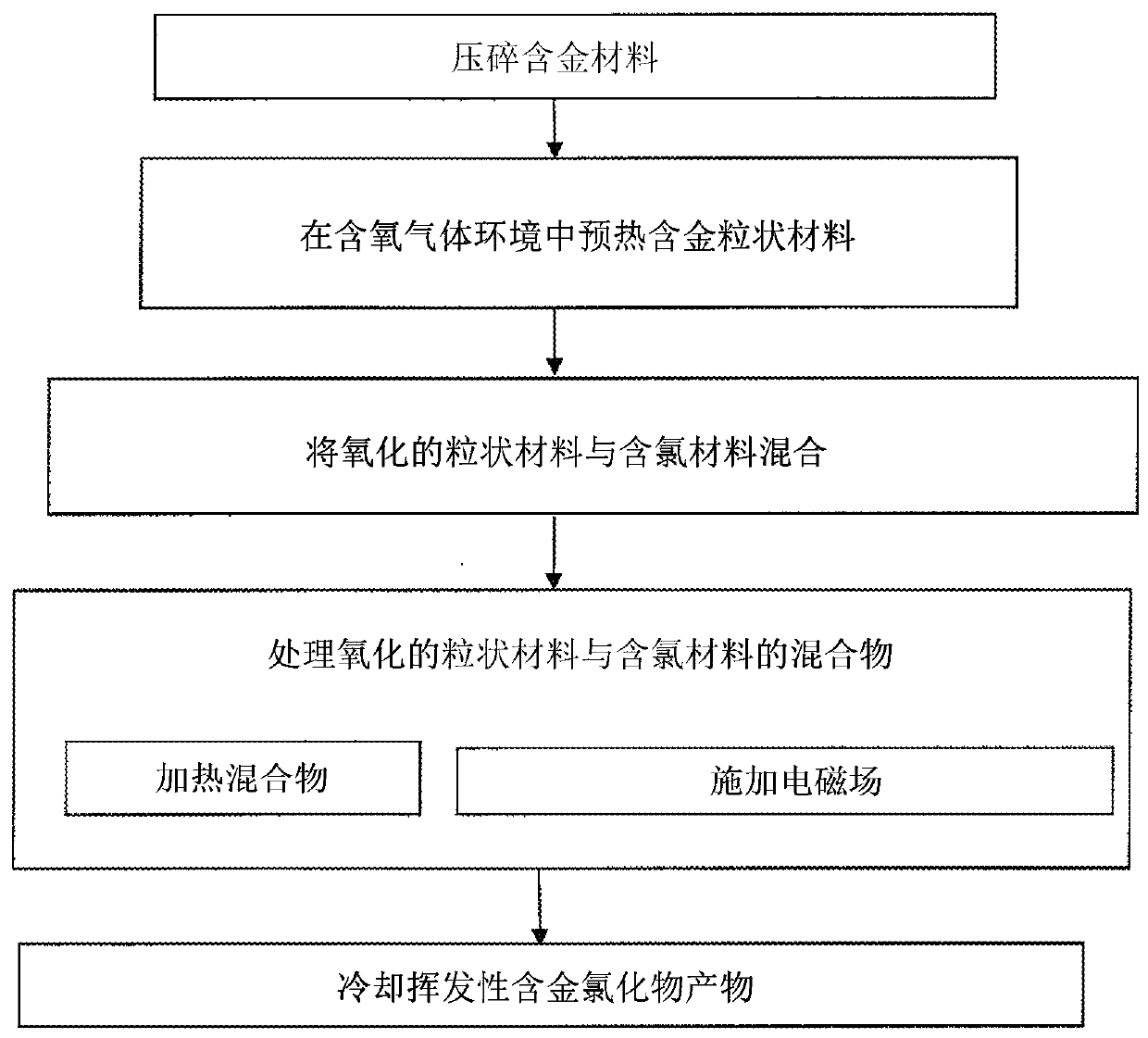
[0093] Gold recovery from gold-bearing materials
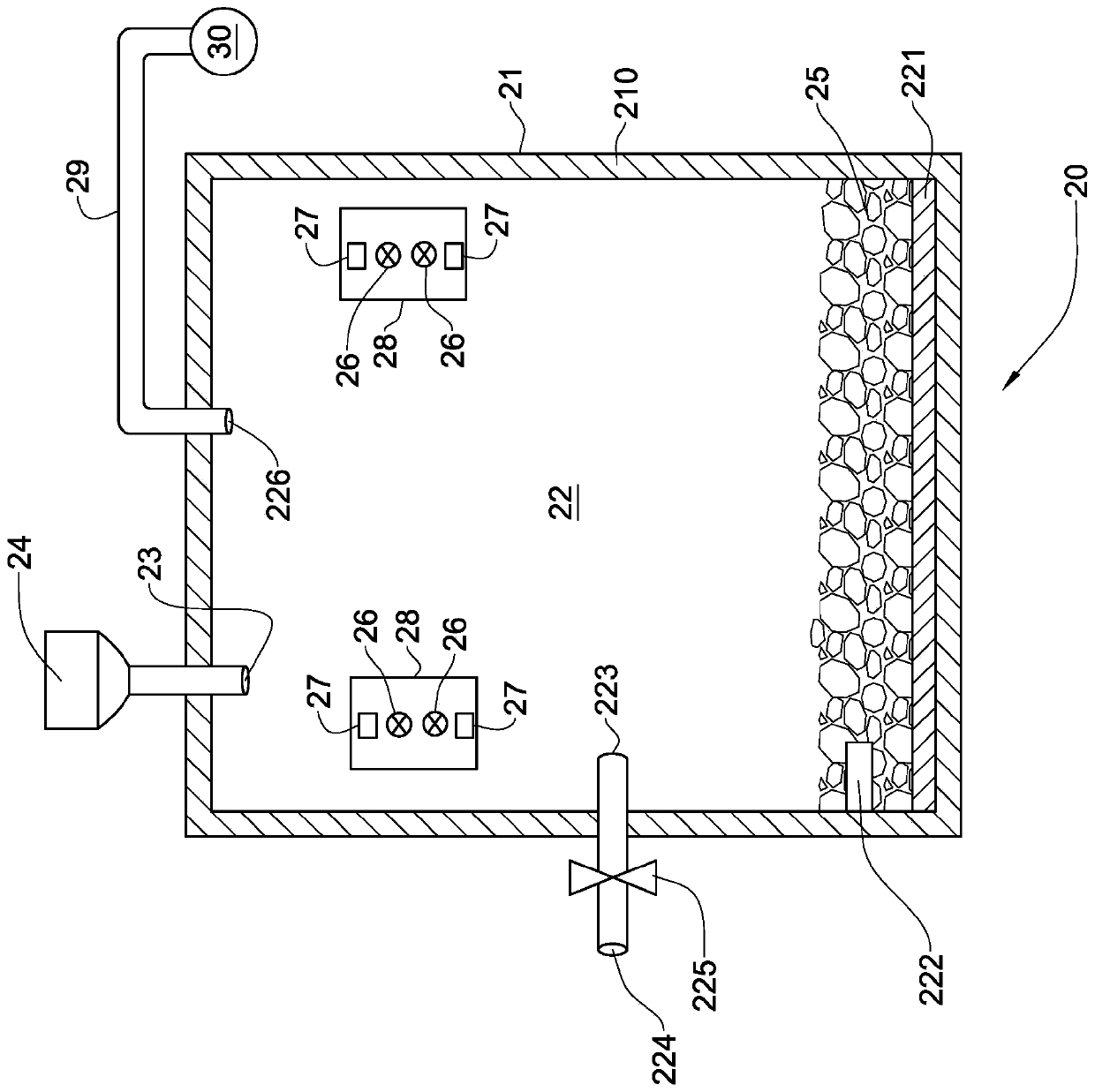
[0094] A study of low temperature chlorine sintering of various industrial samples with 4-12ppm gold content. Experiments were carried out in alumina crucibles at 200-350° C. in a furnace with heating elements (FeCrAl) from Kanthal. The initial sample was 60 g. Chlorine gas is used as the chlorinated reagent and is fed into the furnace in considerable excess. Chlorine consumption is 10ml / min. The duration of the experiment was 1 hour. For experimental reproducibility, each experiment was performed twice. Sublimed products are removed from the reaction zone by gas flow.
[0095] The samples obtained after sintering were analyzed by ICP-MS spectroscopy. The yield of gold extraction from the product free of arsenic and antimony (0.05% As, 0.003% Sb) was 80-90%. Sublimation of non-ferrous metals (Cu, Pb, Ag, Zn) and iron was not detected.
example 2
[0097] Recovery of gold from materials containing arsenic, antimony and gold
[0098] It has been found that the presence of arsenic, antimony or combinations thereof in gold-bearing materials drastically reduces gold recovery from 90% to 30-40%.
[0099] To recover high levels of gold, arsenic and antimony are first removed from the product. The second stage of the process must be a selective gold extraction process as described in Example 1.
[0100] During sintering of materials containing arsenic and antimony (0.6% As, 0.3% Sb) a very high rate of arsenic and antimony sublimation (70-75%) was obtained. At the same time, gold extraction was drastically reduced to 30-40%.
[0101] To prevent the sublimation of arsenic and antimony, preliminary studies have been carried out to remove arsenic and antimony from the starting materials beforehand. Thermodynamic calculations were performed for all reactions. The pre-ground initial sample is 60g (As-0.71%, Sb-0.65%, Cu-11%, Zn-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com