In-vitro culture, induction, activation and cryopreservation method and cell bank establishment of immune cells
A technology of immune cells and cryopreservation method, applied in the biological field, can solve the problems of natural killer cell culture, induction, activation, cryopreservation methods need to be improved, the amplification multiple and tumor killing effect are not good, and the culture system has safety risks. , to meet the needs of a large number of immune cells, good cell viability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
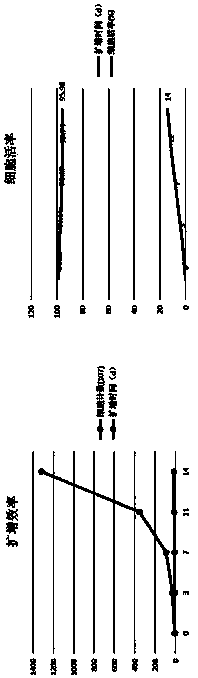
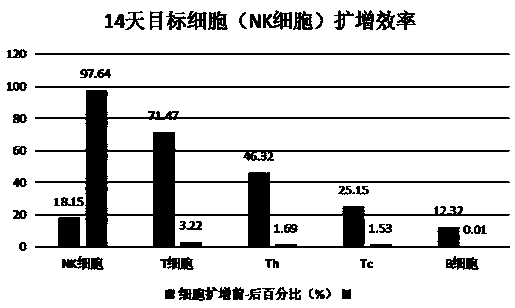
[0033] Using the immune cell culture, induction, activation and expansion method of the present invention, the isolated mononuclear cells are induced, activated and expanded into target immune cells (NK cells), and the activity of the target immune cells is detected.
[0034] 1. Experimental method
[0035] 1. Donor Screening
[0036] 1.1 The hospital is required to sign an informed consent form with the donor in triplicate. One for the donor and one for the medical institution, and one for the laboratory along with the specimen.
[0037] 1.2 The hospital asks for the donor's personal information, past treatment history, family genetic history, and whether there is a history of infectious diseases and abnormal conditions of the hematopoietic or immune system by means of inquiries and filling in forms. The hospital is required to obtain the consent of the donor himself or his authorized personnel to check the medical examination data and obtain the medical examination informa...
Embodiment 2
[0081] The NK cells induced, activated and expanded in vitro in Example 1 were cryopreserved, and the effects of cryopreservation were compared.
[0082] The NK cells obtained on the 14th day of in vitro induction and expansion in Example 1 were cryopreserved using the following four cryopreservation solutions, and the components of the four cryopreservation solutions were as follows:
[0083] Freezing solution 1: 5% by volume of DMSO, 1% by volume of albumin, 1% by volume of aminoethanol, and 93% by volume of X-VOVO15 serum-free medium;
[0084] Freezing solution 2: 5% by volume of DMSO, 2% by volume of albumin, 2% by volume of aminoethanol, and 91% by volume of X-VOVO15 serum-free medium;
[0085] Freezing solution 3: 5% by volume of DMSO, 2% by volume of albumin, and 93% by volume of X-VOVO15 serum-free medium;
[0086] Freezing solution 4: 5% by volume of DMSO, 2% by volume of aminoethanol, 93% by volume of X-VOVO15 serum-free medium;
[0087] Freezing solution 5: 5 volu...
Embodiment 3
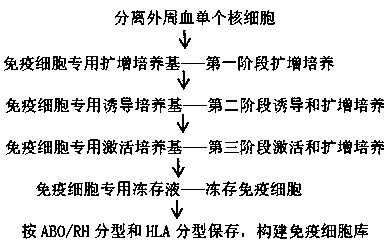
[0096] Using the method for culturing, inducing, and activating immune cells in the embodiment of the present invention, the isolated mononuclear cells are induced and expanded into immune cells, stored according to ABO / RH typing and HLA typing, and immune cell information available for retrieval is established Archives, Construction of immune cell banks.
[0097] 1. Donor Screening
[0098] The hospital asks for the donor's personal information, past treatment history, family genetic history, and whether there is a history of infectious diseases and abnormal conditions of the hematopoietic or immune system by means of inquiries and filling in forms. The hospital must sign an informed consent form with the donor to obtain the consent of the donor himself or his authorized personnel, to check the medical examination data and obtain the medical examination information. Donor physical examination information should include the following items: HIV-1 / 2 antibody, HBsAg, anti-HCV, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com