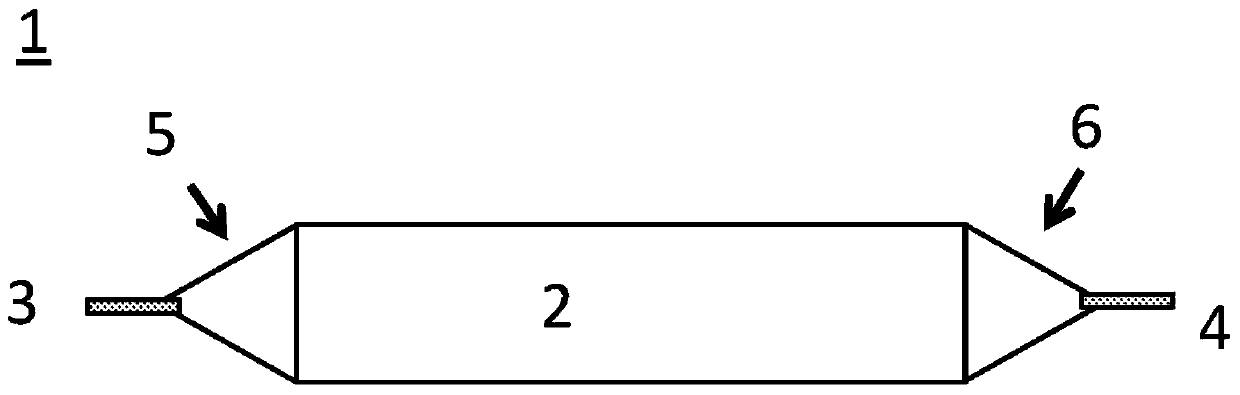
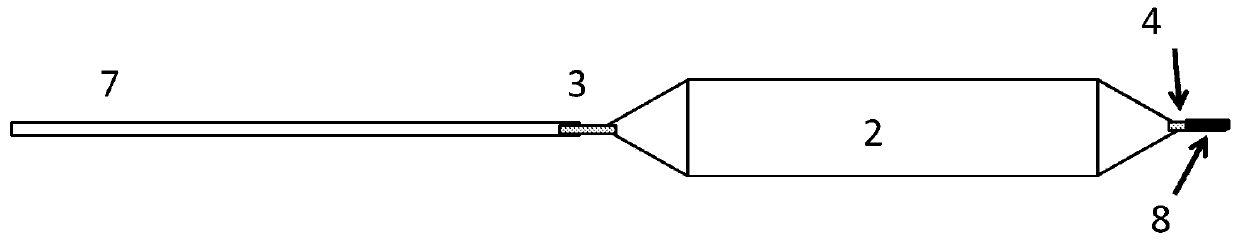
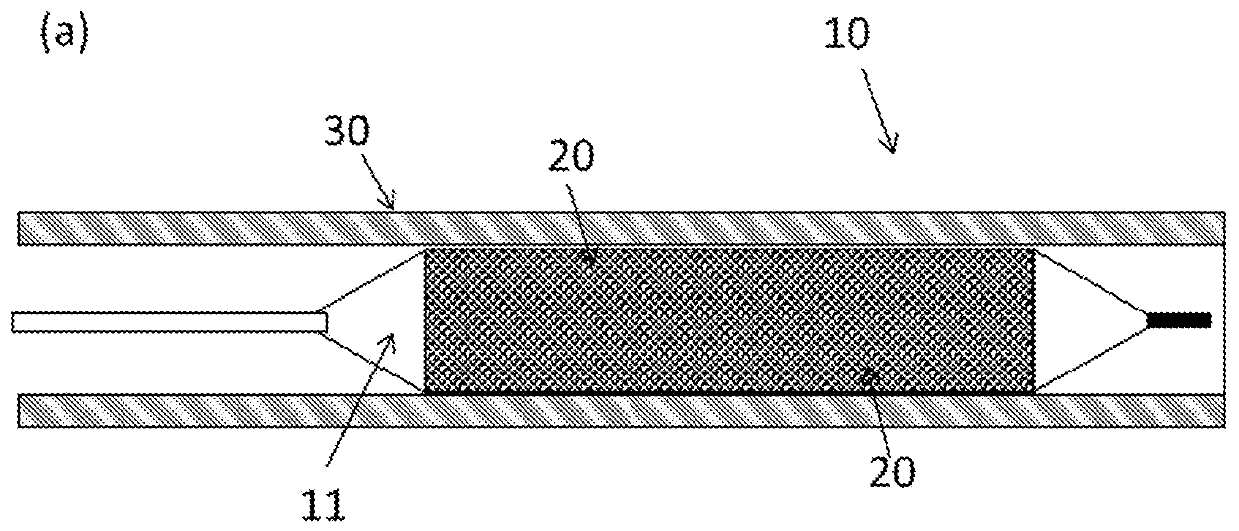
Drug delivery balloon catheter
A balloon catheter and balloon technology, applied in the field of drug-eluting balloons, can solve the problems of uncontrollable, unpredictable, insufficient drug amount, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] Transport loss / travel loss
[0265] Use the same drug coating formula and coating method to coat the test object.
[0266] Test object
[0267] Device A employs a Figure 6b Similar drug delivery systems, the distal end of the outer cannula is as follows Figure 11b The external expansion shown.
[0268] The drug delivery device of device B is a common balloon catheter (DEB) without an outer sheath.
[0269] experimental method
[0270] The in vitro test method was adapted from Seidlitz et al. In Vitro Assay of Drug Transfer from Drug-Coated Balloons (2013), PLoS ONE8(12):e83992 (doi:10.1371 / journal.pone.0083992).
[0271] The following adjustments were made:
[0272] ·Silicone tube is used to simulate the blood vessel wall.
[0273] • The simulated vessel wall was not imaged, therefore the balloon was not fluorescently treated.
[0274] • The drug components were extracted from acetonitrile and analyzed at 227nm using a UV spectrometer.
[0275] • The balloon ...
experiment example 2
[0281] sheath loss
[0282] Use the same drug coating formula and coating method to coat the test object.
[0283] Test object
[0284] Device C employs a Figure 6b Similar drug delivery systems, the distal end of the outer cannula is as follows Figure 11a straight as shown.
[0285] Device D employs a Figure 6b Similar drug delivery systems, the distal end of the outer cannula is as follows Figure 11b The external expansion shown.
[0286] experimental method
[0287] The in vitro test method was adapted from Seidlitz et al. In Vitro Assay of Drug Transfer from Drug-Coated Balloons (2013), PLoS ONE8(12):e83992 (doi:10.1371 / journal.pone.0083992).
[0288] The following changes were made:
[0289] ·Silicone tube is used to simulate the blood vessel wall.
[0290] • The simulated vessel wall was not imaged, therefore the balloon was not fluorescently treated.
[0291] • The drug components were extracted from acetonitrile and analyzed at 227nm using a UV spectromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com