Application of near-infrared weightless atom Bodipy to photodynamic therapy of metastatic tumor and upconversion
A technology for photodynamic therapy and fluoroboropyrrole, which is applied in the field of fluoroboropyrrole-based photodynamic therapy reagents for tumor/metastasis tumors and up-conversion reagents, photodynamic therapy reagents and up-conversion reagents, and can solve the problem of weak up-conversion luminescence, weak up-conversion luminescence, and up-conversion reagents. The toxicity and biosafety of inorganic upconversion nanoparticles are unclear, and the upconversion efficiency is low, which can achieve the effect of high-efficiency photodynamic therapy, good tissue penetration, and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] [Example 1] Preparation of BDP-helix
[0059] (1) Synthetic references of the compounds were synthesized. [16] Mix fluoroborate pyrrole dye 1 (100mg, 0.175mmol) containing iodine atom at the 2 and 6 positions with o-formylphenyl boronate 2 (80mg, 0.52mmol), add tetrabutylammonium bromide (60mg, 0.17mmol) As a phase transfer catalyst, it was dissolved in 20 mL of tetrahydrofuran solvent under the protection of inert gas, and tetrakis(triphenylphosphine) palladium catalyst (0.017-0.035 mmol) was added. 6 mL of 2M aqueous sodium carbonate solution was added, and the reaction was heated at 80° C. for 1.5 h (monitoring the progress of the reaction by TLC to avoid deterioration of the product). After the reaction, the reaction solution was cooled to room temperature, and the solvent was distilled off. Then an organic solvent of dichloromethane was added, washed with saturated brine (3×20 mL), and dried over anhydrous magnesium sulfate or anhydrous sodium sulfate. Separatio...
Embodiment 2
[0061] [Example 2] Preparation of BDP-helix-NP
[0062] (1) Preparation of polymer PSMA-PEG-OA: PSMA (160mg) and PEG 2000 -NH 2 (200 mg) was dissolved in 30 mL of dry tetrahydrofuran solvent. Heated at 70°C for 5h under nitrogen protection. Octadecylamine (26 mg) was dissolved in 5 mL of dry THF, and slowly added to the above mixture within 30 min. Continue stirring at 70°C for 5h. After the reaction, the reaction solution was stored at a low temperature at 4°C.
[0063]
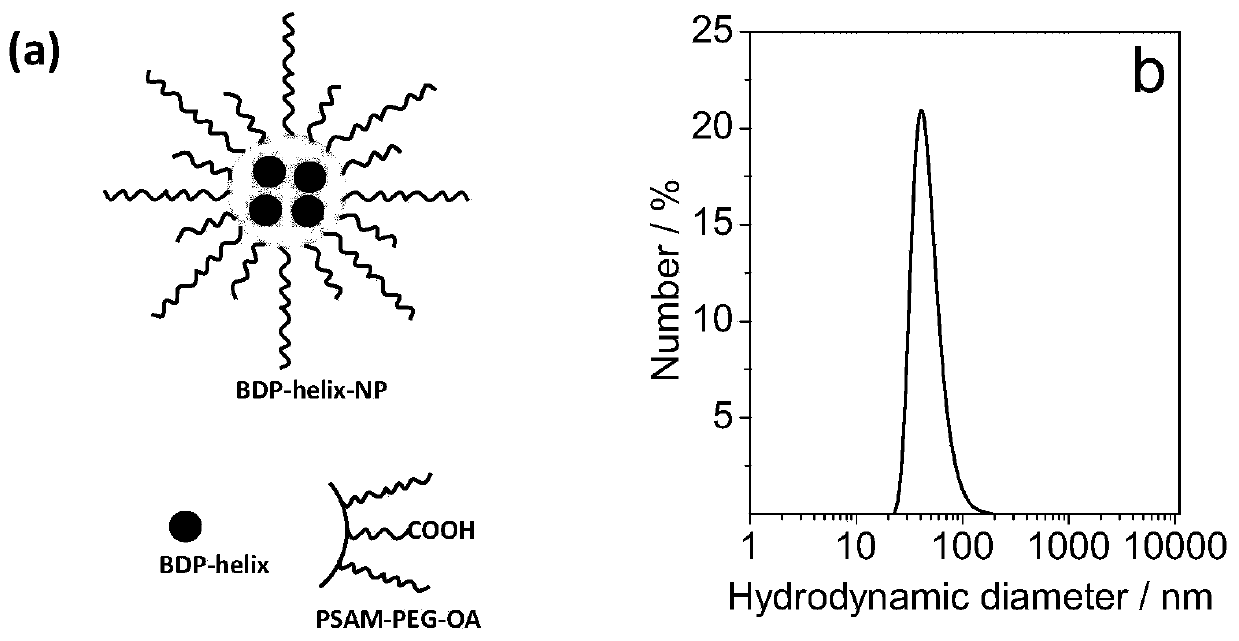
[0064] (2) Dissolve 0.5 mg of BDP-helix and 30 mg of PSMA-PEG-OA in 2 mL of THF, and add 10 mL of PBS buffer solution (pH=7.24). The mixture was stirred at 40 °C for 2 h. After the reaction solution was cooled to room temperature, it was dialyzed with deionized water for 24h to obtain BDP-helix-NP (such as figure 1 Shown in a) Store at 4°C at low temperature.
[0065] figure 1 In b is the dynamic light scattering test result of the BDP-helix-NP of the embodiment, and the test result shows that th...
Embodiment 3
[0066] [Example 3] Determination of BDP-helix-NP's ability to sensitize singlet oxygen and determination of cytotoxicity
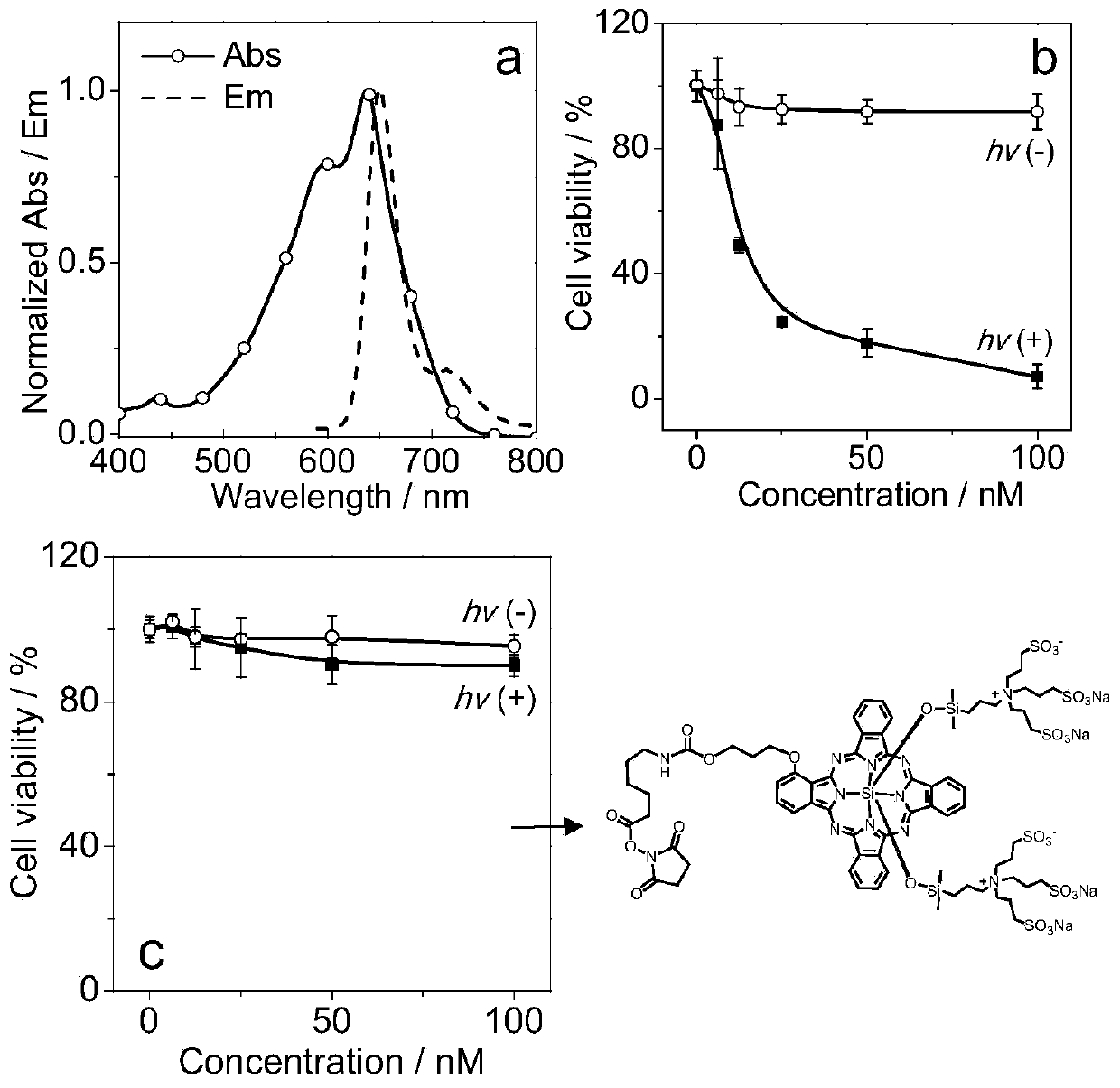
[0067] (1) Ability to sensitize singlet oxygen: 10 5 CT26 tumor cells were cultured in a cell culture dish. After 12 hours of culture, a mixture of BDP-helix-NP (100nM, 2mL PBS buffer) and singlet oxygen fluorescent probe (SOSG, 5μM) was added, and the culture was continued for 4 hours. LED light source (20mW / cm 2 ) after 5min of light, the laser (λ ex = 488 nm) to detect changes in SOSG fluorescence. Use methylene blue as a standard reference compound (the singlet oxygen quantum yield of methylene blue in water is 60% [17] ), the measured singlet oxygen quantum yield of BDP-helix-NP (Φ Δ ) is 21%.
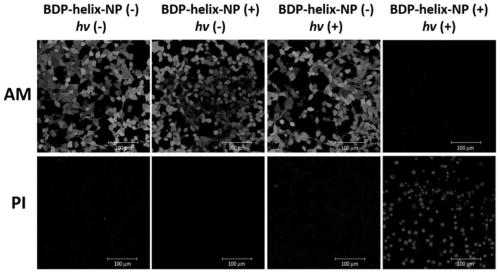
[0068] (2) In vitro cytotoxicity assay: the tumor cell CT26 cells were cultured on a 96-well plate, and BDP-helix-NP (0, 6.25, 12.5, 25, 50, 100 nM) were added after 24 hours, and the cells were incubated at 37°C and 5% CO 2 The incubation was continued ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap