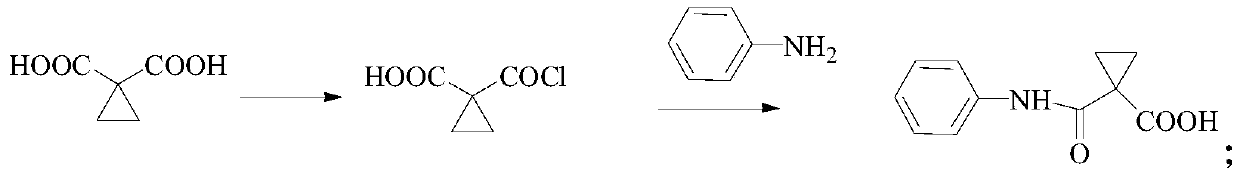Preparation method of cabozantinib defluorination impurity
A technology for cabozantinib and impurities, which is applied in the field of drug preparation and can solve the problems of complex structural formula of cabozantinib, long synthetic route steps, and many potential impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The technical solutions of the present invention will be further described below through the accompanying drawings and embodiments.
[0021] The invention provides a method for preparing cabozantinib defluorinated impurities, comprising the following steps:
[0022] S1. Preparation of Intermediate 1: First, add cyclomalonic acid and ethyl acetate to the reactor, add triethylamine when the temperature is lower than 5°C, control the temperature to less than 10°C, react for 1 hour, and then control the temperature to less than 10°C Add thionyl chloride dropwise, react for 1 hour, add aniline when the temperature is less than 10°C, and react for 16 hours. PE / 1L TBME is beaten, filtered and dried to obtain intermediate 1, and the specific reaction formula is:
[0023]
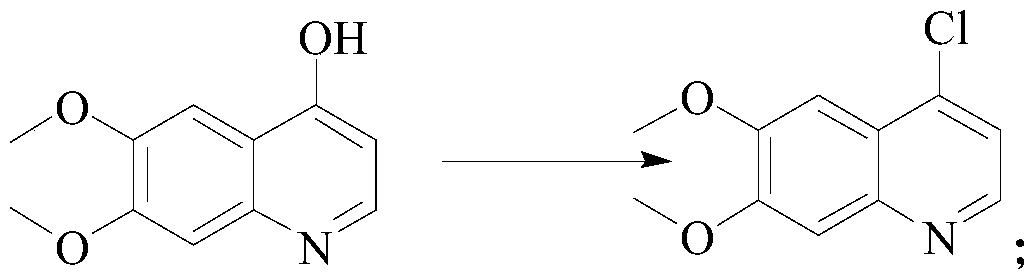
[0024] Preparation of S2, 4-chloro-6,7-dimethoxyquinoline: 4-hydroxyl-6,7-dimethoxyquinoline, acetonitrile, phosphorus oxychloride are added to the reactor successively, and the temperature is raised to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com