Synthesis method of cabozantinib dimer
A technology of cabozantinib dimer and synthesis method, which is applied in the field of cabozantinib dimer synthesis, and can solve the problems of complex structure of cabozantinib, many potential impurities, long synthetic route steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The technical solutions of the present invention will be further described below through the drawings and embodiments.
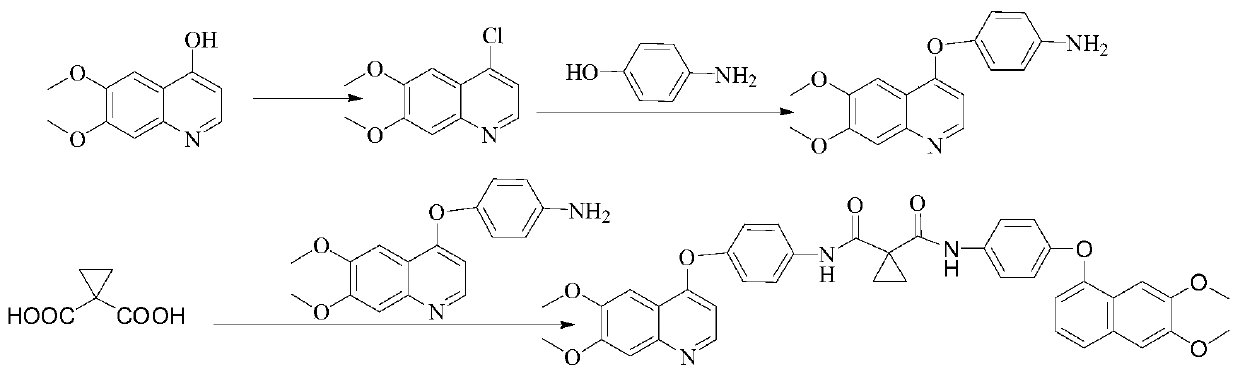
[0019] The invention provides a method for synthesizing cabozantinib dimer, which includes the following steps:
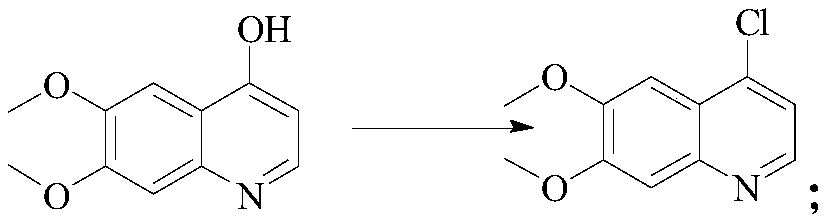
[0020] Preparation of S1, 4-chloro-6, 7-dimethoxyquinoline: add 4-hydroxy-6, 7-dimethoxyquinoline, acetonitrile and phosphorus oxychloride to the reactor in turn, and heat to React at 75°C for 24h. After the reaction is complete, it is evaporated to dryness and poured into ice water. Adjust PH=7 with alkali and ammonia as alkali. Extract and evaporate to dryness with DCM. Beat and filter with 1.5L PE / 1.5L TBME to obtain 4-chloro-6. 7-Dimethoxyquinoline, the specific reaction formula is:
[0021]
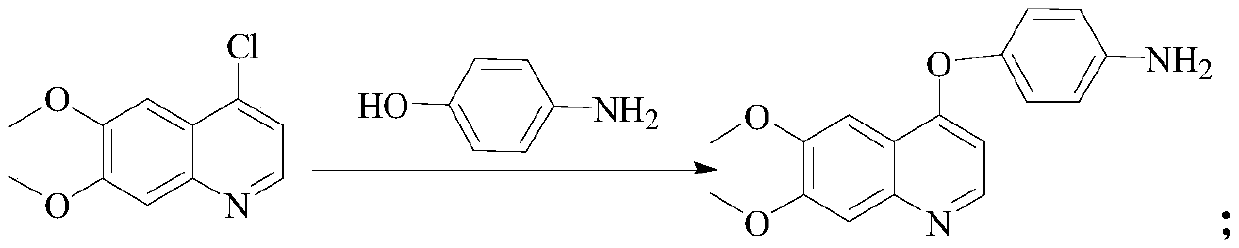
[0022] S2. Preparation of Intermediate 1: Add organic solvent and base to the reactor and stir for 10 min. Organic solvent is DMSO and base is potassium tert-butoxide. After adding p-hydroxyaniline and stirring for 20 min, add 4-chloro-6, 7- Dimethoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com