Synthesis method of febuxostat decarboxylation impurity
A febuxostat and synthetic method technology, applied in the field of chemical pharmacy, can solve the problems of febuxostat with complex structural formula, many potential impurities in febuxostat API, long steps in the synthetic route, etc., and achieve high product yield and reliable Controllable, easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Embodiments of the present invention will be further described below.
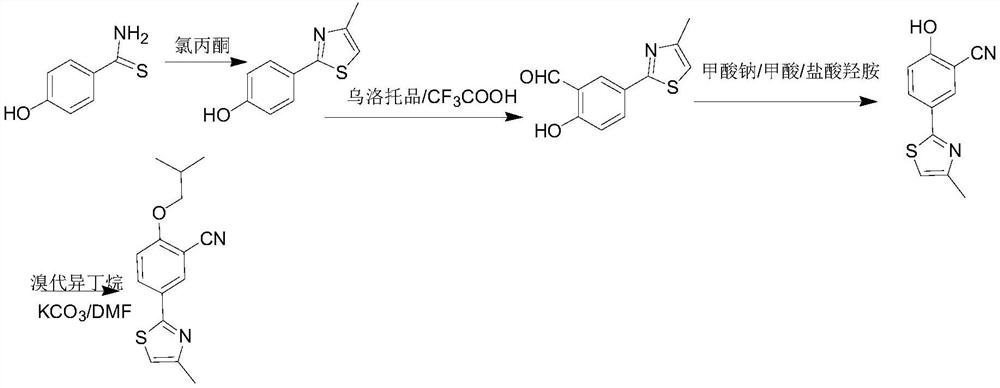
[0025] A kind of synthetic method of febuxostat decarboxylation impurity, reaction route is as follows:
[0026]
[0027] Among them, the specific content is as follows:
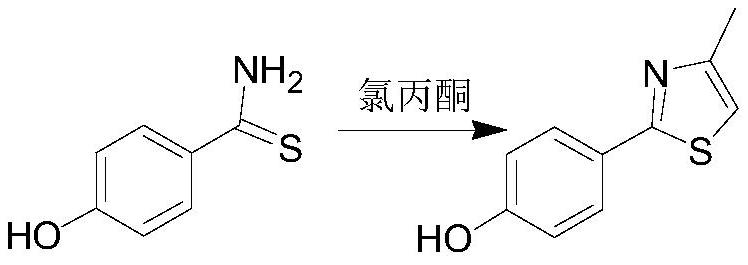
[0028] (1) Preparation of 2-(4-hydroxyphenyl)-4-methylthiazole:
[0029]
[0030] During the reaction, 2.26g of p-hydroxythiobenzamide and 15mL of absolute ethanol were placed in a 100mL three-necked flask, 2.42g of 1-chloroacetone was added under stirring, heated to reflux, and a solid was precipitated, reacted for 4 hours, TLC (chloroform: Ethanol=9:1) detection control reaction end point. After the reaction was completed, it was cooled and filtered to obtain 1.72 g of solid, with a yield of 99.5%.
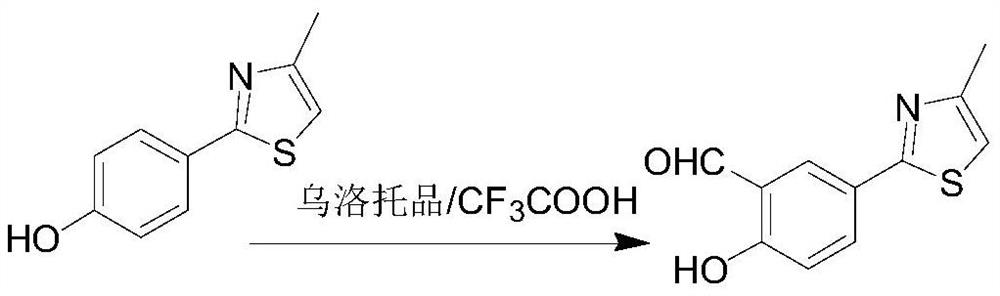
[0031] (2) Preparation of 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole
[0032]
[0033] During the reaction, 1.71kg of 2-(4-hydroxyphenyl)-4-methylthiazole was placed in a 20L three-necked flask, 15L of trifluoroaceti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com