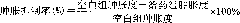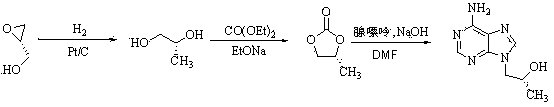Patents
Literature
34 results about "Chloracetone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
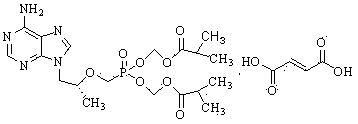
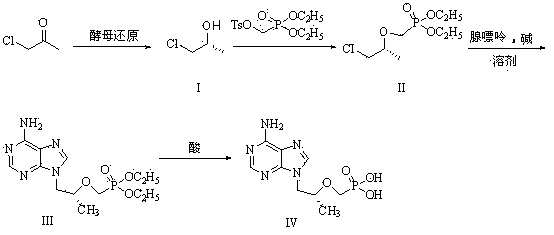
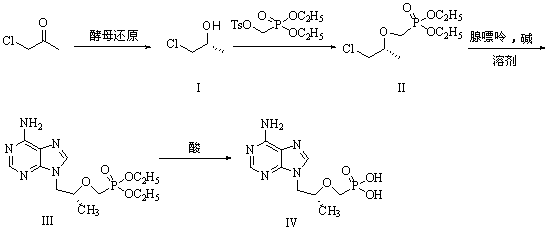
Method for synthesis of PMPA by combining biological technique and chemical technique
ActiveCN102899367AHigh yield of reductionEasy post-processingGroup 5/15 element organic compoundsMicroorganism based processesBiotechnologyDiethyl phosphate
Belonging to the technical field of medicine synthesis, the invention relates to a method for synthesis of PMPA by combining a biological technique and a chemical technique. The method comprises: taking chlorinated acetone as a starting raw material, conducting yeast fermentation reduction so as to obtain chiral chloropropanol (I), then subjecting the chiral chloropropanol (I) and diethyl p-toluenesulfonyloxyphosphonate to a condensation reaction under the action of alkali so as to obtain a reaction intermediate (II); preparing R-9-(2-hydroxypropyl)adenine (III) from adenine and the reaction intermediate (II); and hydrolyzing the obtained R-9[2-(diethylphosphonomethoxy)propyl]purine, thus obtaining the PMPA (IV). The method combines the biological technique and the chemical technique and obtains a key chiral alcohol by means of a biological means, i.e. fermentation. The method has the characteristics of short reaction process, short reaction time, high mass yield, and can produce products with good quality, thus being suitable for industrialized production.
Owner:黄石福尔泰医药科技有限公司
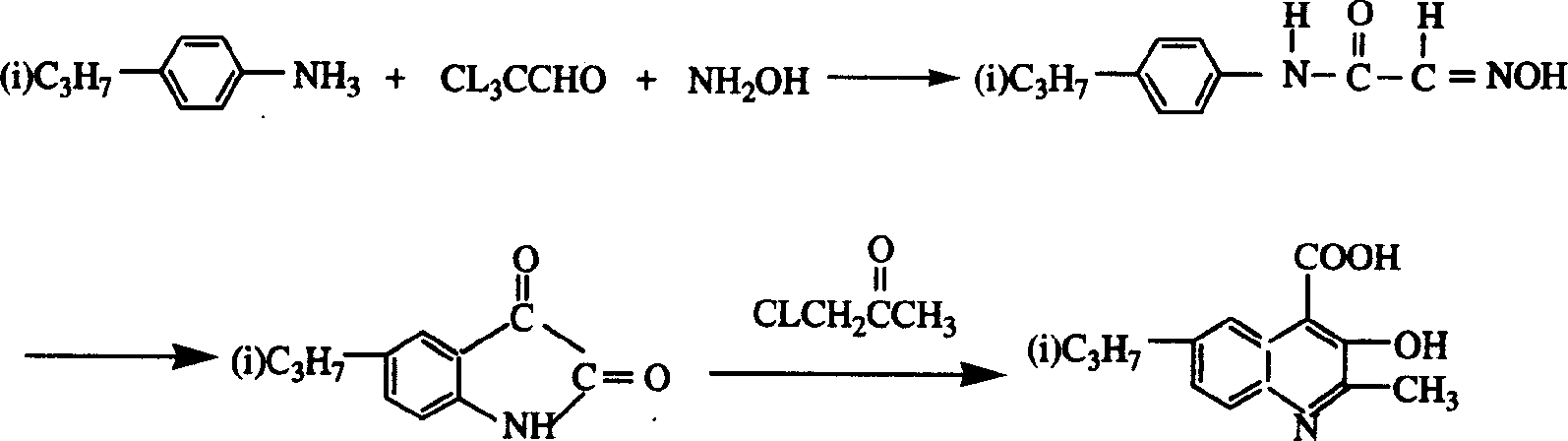
2-methyl-3-hydroxy-6-isopropylquinoline-4-carboxyl acid preparation method
The preparation method for 2- methyl -3-hydroxyl -6- isopropylquinoline-4- hydroxy acid, which comprises: adding chloral and inorganic salt liquid into reaction bulb, as well as p-isopropoxyphenylamine and strong hydrochloric acid and hydroxylamine hydrochloride, obtaining 4-isopropyloximinoacetamide solid; adding strong sulphuric acid, heating, stirring, cooling; pouring reaction liquid into ice water, filtering to obtain 5- isopropyl-isatin solid; with calcium oxide or calcium hydroxide, adding 5- isopropyl-isatin solid into reaction bulb, heating, dropping chloracetone; then neutralizing with acid to obtain golden-ocher product. This invention is simple and security.
Owner:CHINA LUCKY FILM CORP +1
Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof
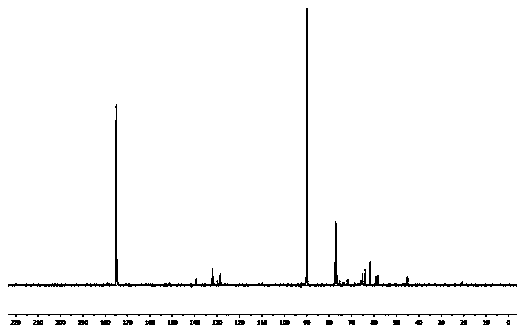
The invention relates to compounds. Cyanoethyl acetate and chloroacetone with different substituent groups are used as initial raw materials, a cyanoethyl ester compound is generated under the action of sodium ethoxide, then furan compounds are generated through intramolecular ring closing under the action of trifluoroacetic acid, the furan compounds respectively react with 2-pyrrolidone, valerolactam and caprolactam under the action of phosphorus oxychloride to form a ring, so as to obtain furo[2, 3-d]pyrimidinone compounds, and then oxygen-sulfur exchange is performed under the action of phosphorus pentasulfide, so as to obtain the furo[2, 3-d]pyrimidinethione compounds. The 54 kinds of obtained tricyclic furo[2, 3-d]pyrimidone compounds are used as positive control of DOX, the inhibitory activity of Hela cervical cancer cells, MCF-7 breast cancer cells and A549 lung cancer cells is achieved, and results show that the compounds C11 and C17 have the inhibitory activity of the Hela cervical cancer cells; the compounds D5 and D7 have inhibitory activity on A549 lung cancer cells; and the compound C14 has inhibitory activity on MCF-7 breast cancer cells.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Method for preparing deuterated chloroform by using hexachloroacetone as intermediate
InactiveCN103408413ASolve processing problemsAvoid emissionsOrganic compound preparationCarbonyl compound preparationPtru catalystDeuterated chloroform
The invention provides a method for preparing deuterated chloroform by using hexachloroacetone as an intermediate. The method comprises the following steps: step 1, producing hexachloroacetone by monochloroacetone and / or polychlorinated acetone raw material liquid, and comprising the steps of raw material liquid pre-treatment, chlorination catalytic reaction and reaction product purification; step 2, producing deuterated chloroform by taking hexachloroacetone as raw material, and comprising the steps of hydrolytic catalytic reaction and reaction product purification. The method solves the problem of the disposing of waste material produced during the production of monochloroacetone, trichloroacetone and the like, and is in favor of relieving the environment burden; common cheap chemical is used as catalytic agent, the side reaction is less, the reaction product is easy to separate, and the content of the produced pollutant is greatly lowered. Through the utilization of the polarization and weakening effects of connecting bond of the carbonyl carbon of the hexachloroacetone and trichloromethyl, the carbonyl carbon of the hexachloroacetone and trichloromethyl are hydrolyzed in an alkalescence condition to produce deuterated chloroform, and the reaction product is CO2 that can be discharged into air directly. The method has important social benefits and economic benefits.
Owner:QINGDAO TENGLONG WEIBO TECH
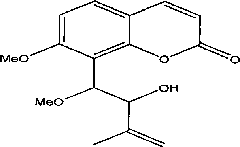
Method for synthesizing (+/-)-Murracarpin and anti-inflammatory and analgesic effect thereof
InactiveCN101824015AMild reaction conditionsEasy to operateOrganic active ingredientsOrganic chemistryEpoxyAcetic anhydride
The invention relates to the field of natural medicinal chemistry, in particular to a method for synthesizing (+ / -)-Murracarpin and anti-inflammatory and analgesic effect thereof. The method for preparing the (+ / -)-Murracarpin comprises the following steps of: reacting 7-hydroxy coumarin, acetic anhydride, trifluoroacetic acid and hexamethylenetetramine to obtain 7-hydroxy-8-formoxyl coumarin; reacting the 7-hydroxy-8-formoxyl coumarin with (Me)2SO4 to obtain 7-methoxy-8-formoxyl coumarin; reacting the obtained 7-methoxy-8-formoxyl coumarin with 1-chloroacetone to obtain 8-(2'-acetyl epoxy ethyl)-7-methoxy coumarin, and reacting the 8-(2'-acetyl epoxy ethyl)-7-methoxy coumarin, methyltriphenyl phosphorous bromide and LDA to obtain 8-(2'-propenyl epoxy ethyl)-7-methoxy coumarin; and performing acid hydrolysis on the obtained 8-(2'-propenyl epoxy ethyl)-7-methoxy coumarin in methanol to obtain the (+ / -)-Murracarpin. Research shows that the obtained compound has anti-inflammatory and analgesic effect.
Owner:许瑞安
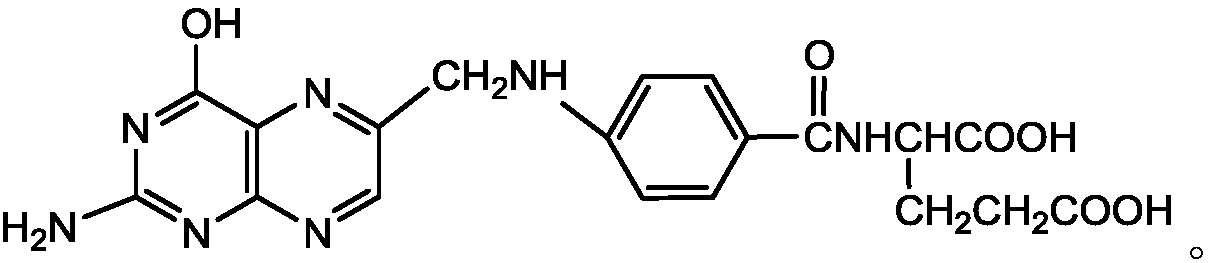
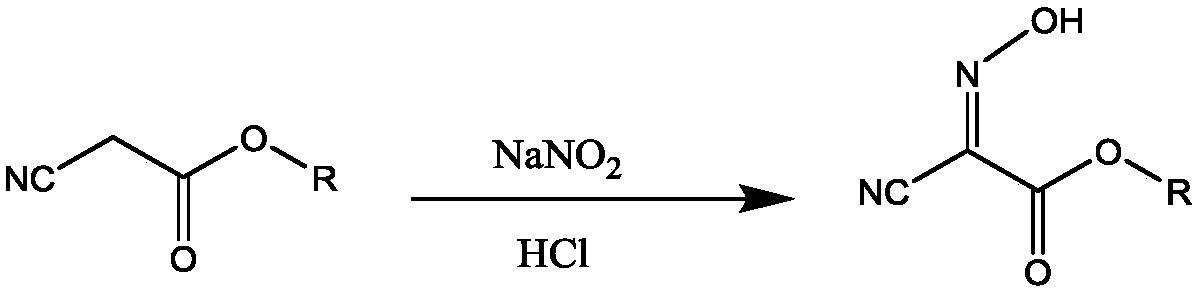
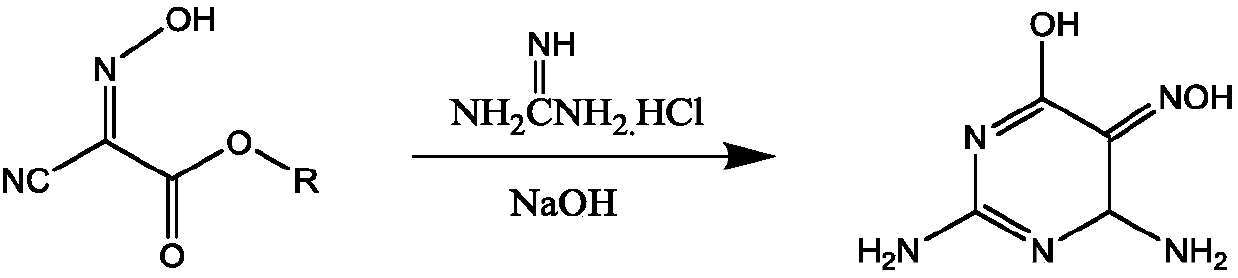
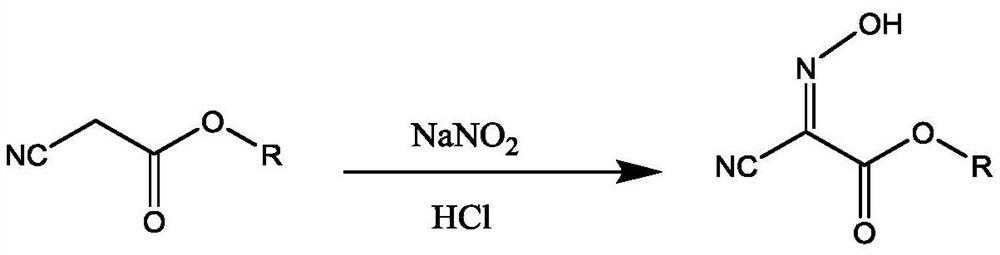
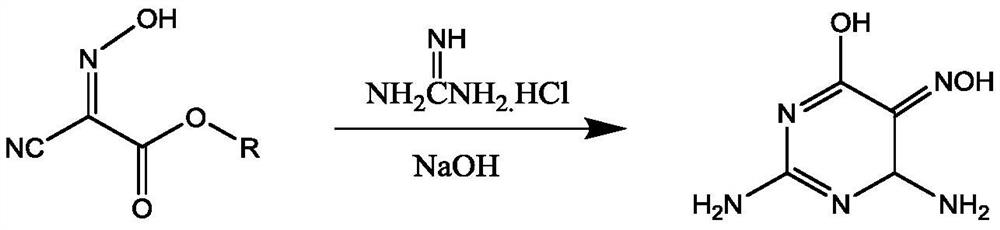
Preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid
ActiveCN109535083AIncrease productionImprove targeting selectivityOrganic chemistryMolecular sieve catalystsSolventAcid substances
The invention relates to a preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid. The preparation method comprises the following steps: enabling cyanoacetate and sodium nitrite to be subjected to reaction under the action of an organic solvent and / or an inorganic solvent and an acidic substance to obtain 2-oximidocyanoacetate; then, enabling the 2-oximidocyanoacetate andguanidine hydrochloride to be subjected to reaction under an alkaline condition to obtain 2,4-diamino-5-isonitroso-6-oxopyrimidine; enabling the 2,4-diamino-5-isonitroso-6-oxopyrimidine and hydrogen gas to be subjected to reaction under an alkaline condition by means of action of Pd / C to obtain 2,4,5-triamino-6-hydroxypyrimidine, and adding sulfuric acid to adjust the pH value to obtain 2,4,5-triamino-6-hydroxypyrimidine sulfate; and then, adding trichloroacetone and N-(4-aminobenzoyl)-L-glutamic acid to be subjected to reaction in a buffer solution under the action of a catalyst molecular sieve so as to obtain the folic acid. Based on the process route, the invention explores the influences of different reaction steps and refining conditions on the preparation route of 2,4,5-triamino-6-hydroxypyrimidine sulfate and the folic acid so as to reduce the wastewater pollution while increasing the yield.
Owner:ZHEJIANG BENLI TECH CO LTD
Synthesis method of febuxostat decarboxylation impurity
InactiveCN112961118ALow costModerate and controllable operationOrganic chemistryTesting medicinal preparationsHexamethylenetetramineReaction step
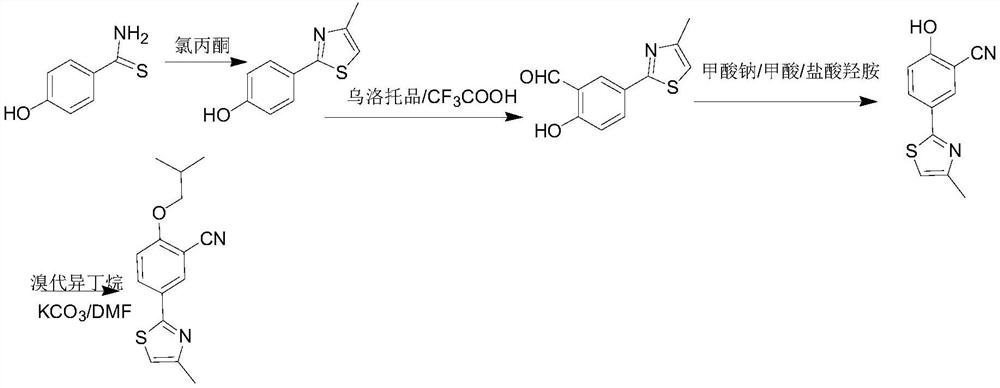
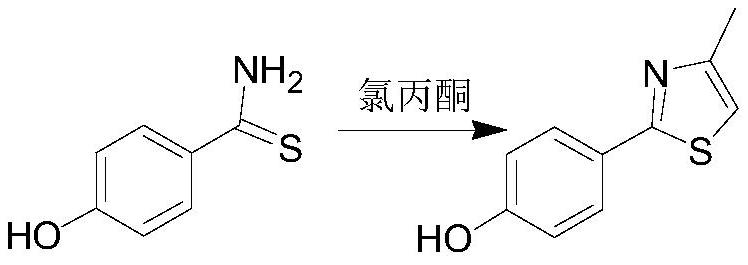
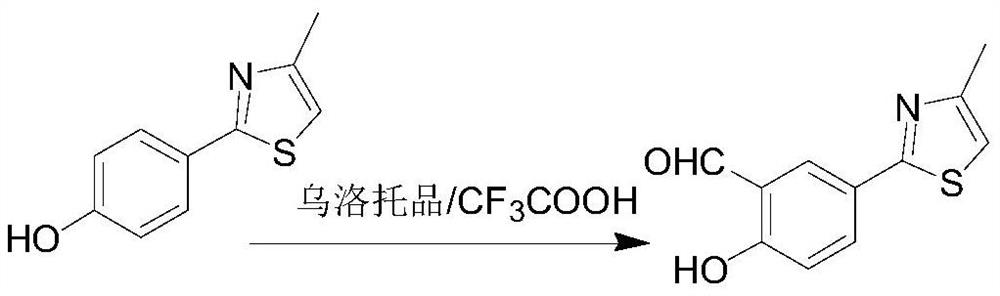
The invention discloses a synthesis method of a febuxostat decarboxylation impurity, which comprises the following steps: by taking p-hydroxybenzenesulfamide as a raw material, carrying out reflux condensation on p-hydroxybenzenesulfamide and chloroacetone in ethanol, and carrying out hydroformylation, cyanation and alkylation on trifluoroacetic acid and urotropine, so as to obtain the febuxostat decarboxylation impurity with the reaction yield of more than 90% in each step. According to the synthesis method of the febuxostat decarboxylation impurity, the reaction steps are mild and controllable, the operation is simple, and the product purity reaches 99% or above.
Owner:LANGFANG NORMAL UNIV
Preparation method of tralopyril
PendingCN113880745AThe synthetic route is simpleRaw materials are easy to obtainOrganic chemistryAlkaneChlorobenzene
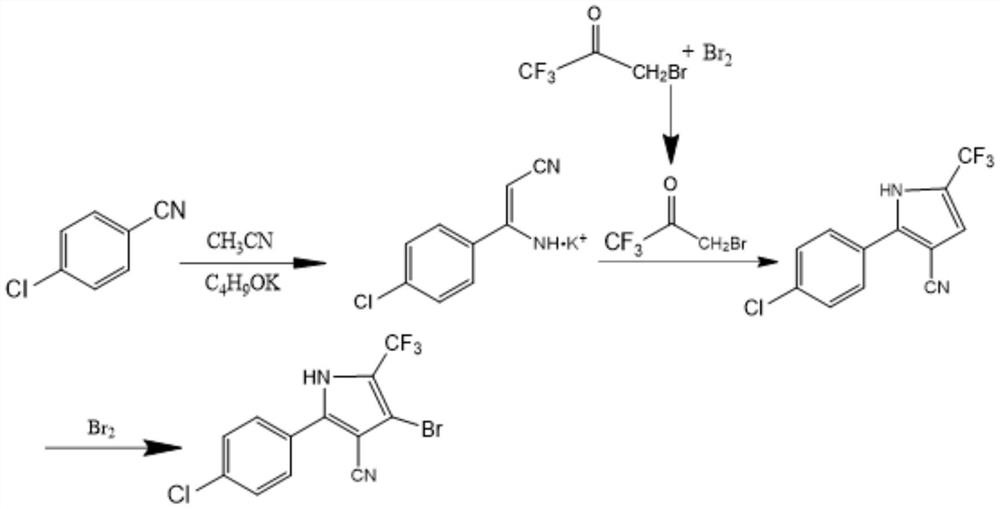
The invention discloses a preparation method of tralopyril, and particularly relates to the technical field of agriculture. The preparation method provided by the invention comprises the steps of 1, dissolving p-chlorobenzonitrile in acetonitrile, and carrying out a salt forming reaction under the catalysis of an organic base to obtain an alkali metal salt (I) of p-chlorophenyl amino acrylonitrile; 2, dissolving 1, 1, 1-trichloroacetone in alkyl chloride, carrying out a one-step bromination reaction with bromine, and carrying out extraction and distillation to obtain 1-bromo-3, 3, 3-trifluoroacetone (II); 3, dissolving the intermediate I in alkane, carrying out a ring closing reaction with the intermediate II through acid catalysis to obtain a 2-(4-chlorphenyl)-5-(trifluoromethyl)-1H-pyrrole-3-nitrile crude product, and carrying out distillation and recrystallization to obtain a pure product; and 4, performing two-step bromination on the intermediate III through bromine to obtain tralopyril. According to the intermediate obtained by the preparation method disclosed by the invention, the final product tralopyril is low in impurity content and high in product purity, the raw materials are simple and easy to obtain, the preparation process is simple, the reaction conditions are mild, and the preparation method is economical and environment-friendly and meets the requirements of industrial mass production.
Owner:山东亿嘉农化有限公司
Method for synthesis of PMPA by combining biological technique and chemical technique
ActiveCN102899367BHigh yield of reductionEasy post-processingGroup 5/15 element organic compoundsMicroorganism based processesBiotechnologyDiethyl phosphate
Belonging to the technical field of medicine synthesis, the invention relates to a method for synthesis of PMPA by combining a biological technique and a chemical technique. The method comprises: taking chlorinated acetone as a starting raw material, conducting yeast fermentation reduction so as to obtain chiral chloropropanol (I), then subjecting the chiral chloropropanol (I) and diethyl p-toluenesulfonyloxyphosphonate to a condensation reaction under the action of alkali so as to obtain a reaction intermediate (II); preparing R-9-(2-hydroxypropyl)adenine (III) from adenine and the reaction intermediate (II); and hydrolyzing the obtained R-9[2-(diethylphosphonomethoxy)propyl]purine, thus obtaining the PMPA (IV). The method combines the biological technique and the chemical technique and obtains a key chiral alcohol by means of a biological means, i.e. fermentation. The method has the characteristics of short reaction process, short reaction time, high mass yield, and can produce products with good quality, thus being suitable for industrialized production.
Owner:黄石福尔泰医药科技有限公司
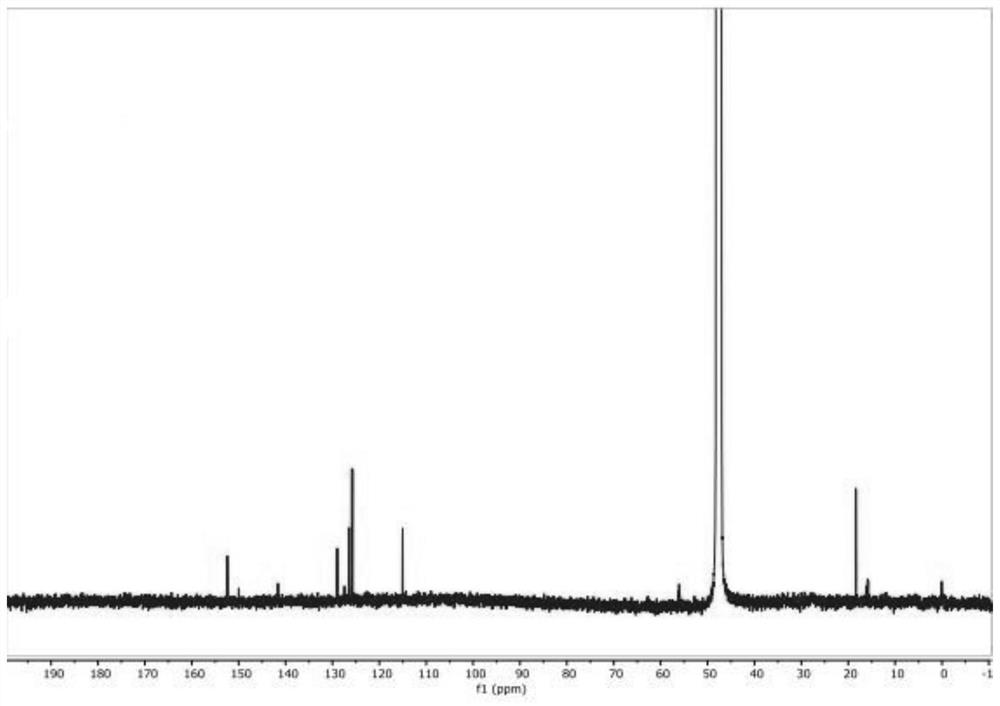
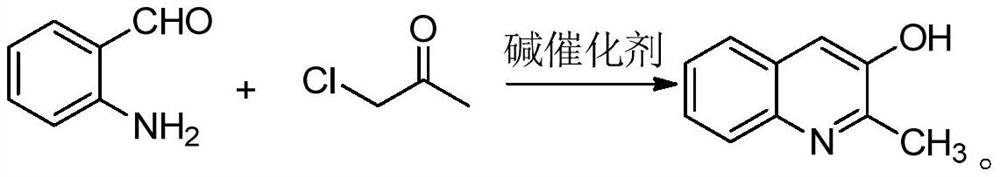
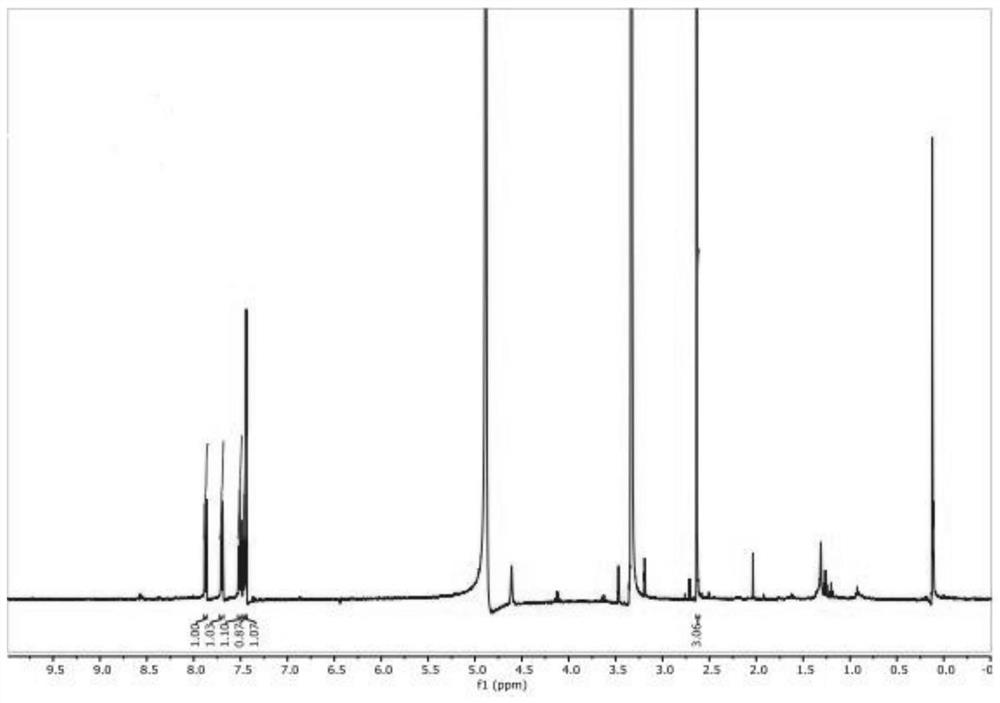
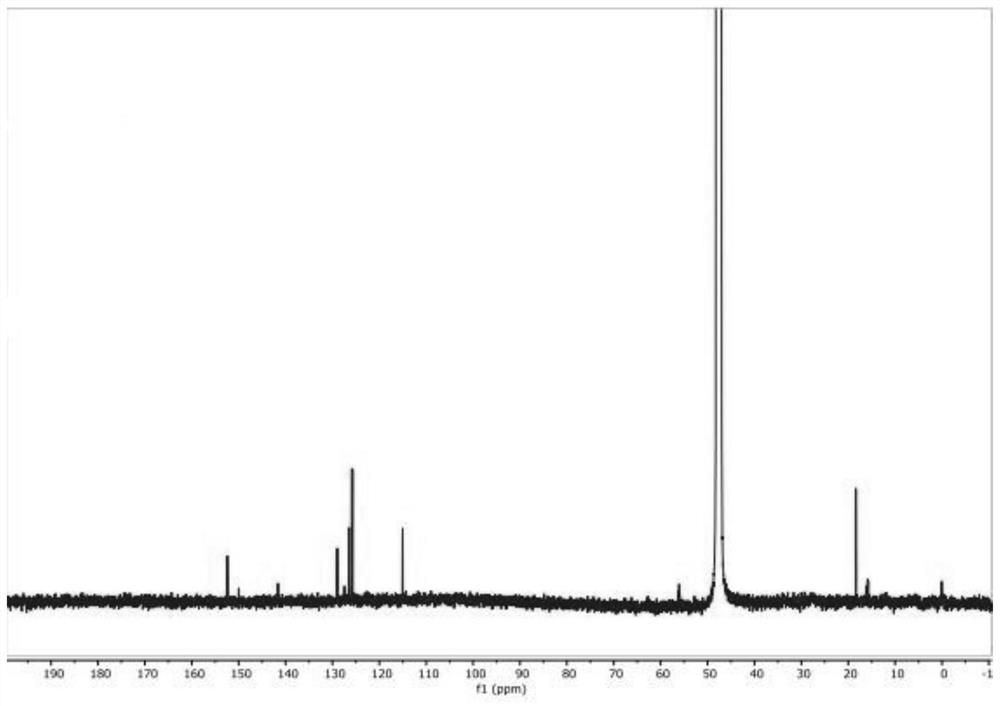
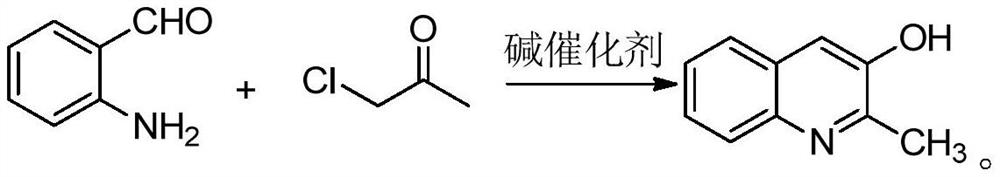
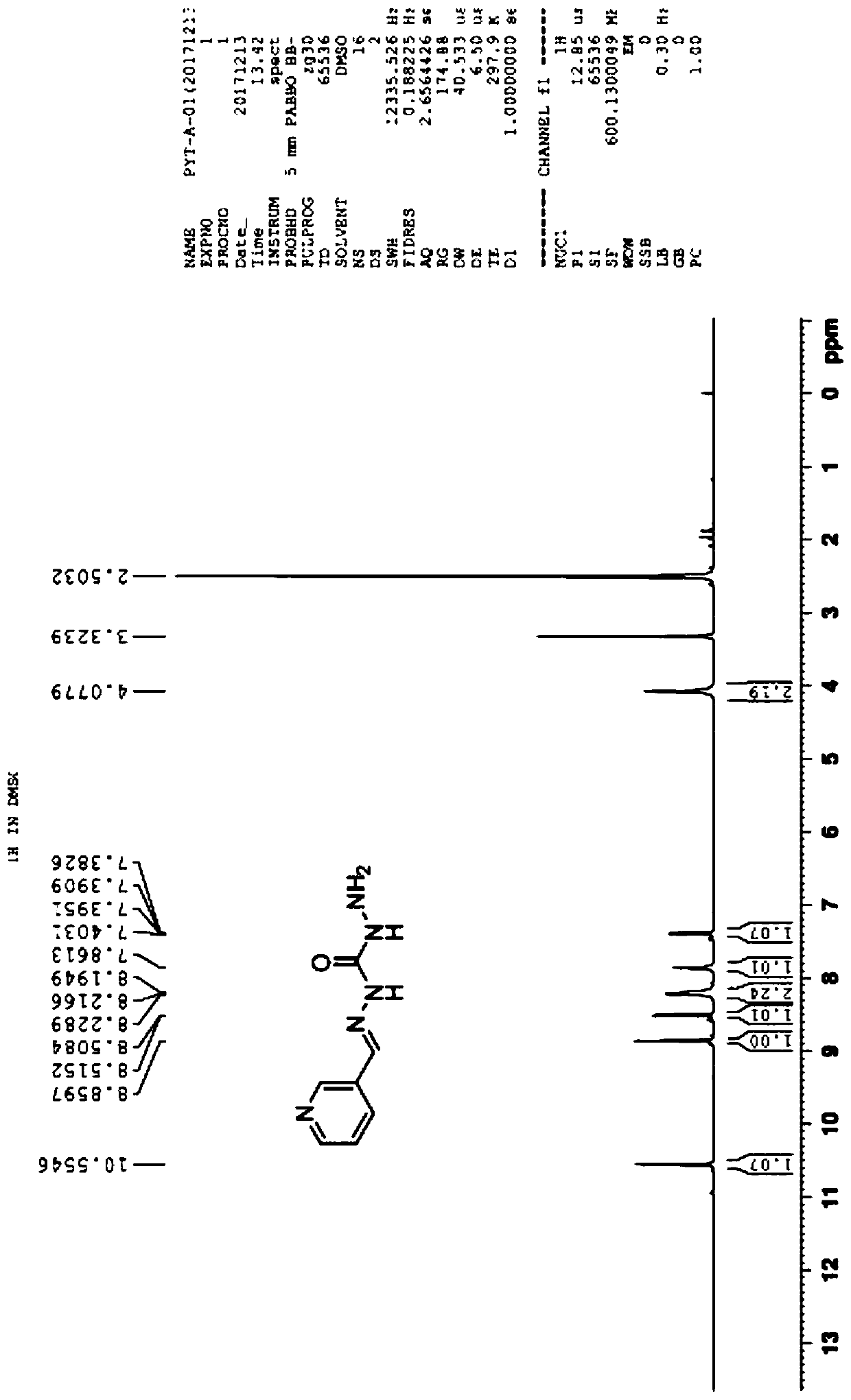
Synthesis method of 2-methyl-3-hydroxyquinoline and preparation method of quinotrione disperse dye
ActiveCN112079773AHigh yieldImprove reaction speedOrganic chemistryAzo dyesDisperse dyePtru catalyst
The invention relates to a synthesis method of 2-methyl-3-hydroxyquinoline and a preparation method of a quinotrione disperse dye. The synthesis method of the 2-methyl- 3-hydroxyquinoline comprises the steps of mixing o-aminobenzaldehyde, chloroacetone, a base catalyst, a phase transfer catalyst and a solvent, adjusting the pH value to 11 to 13, and fully contacting the raw materials under the action of the phase transfer catalyst to obtain a uniformly mixed homogeneous mixed solution with a specific pH value; and then carrying out micro-channel reaction on the mixed solution. In the micro-channel reaction process, reaction materials in the mixed solution is subjected to micron or millimeter-level micro contact reaction, the reaction speed and the reaction selectivity are improved, and theprobability of self-condensation polymerization of o-aminobenzaldehyde or chloroacetone in the micro-channel reaction process is reduced; and therefore, the yield of the 2-methyl-3-hydroxyquinoline is improved.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
The synthetic method of 2-methyl-3-hydroxyquinoline and the preparation method of quinone ketone disperse dye
ActiveCN112079773BHigh yieldImprove reaction speedOrganic chemistryAzo dyesDisperse dyePtru catalyst
The present invention relates to a kind of synthetic method of 2-methyl-3-hydroxyquinoline and the preparation method of quinone disperse dye; in the synthetic method of 2-methyl-3-hydroxyquinoline, first anthranil , chloroacetone, alkali catalyst, phase transfer catalyst and solvent are mixed, and the pH value is adjusted to 11-13. Under the action of the phase transfer catalyst, each raw material is fully contacted to obtain a uniformly mixed homogeneous mixed solution with a specific pH value; Then the mixed solution is subjected to a microchannel reaction. During the microchannel reaction, the reaction materials in the mixed solution can undergo micron or millimeter level micro-contact reactions, which improves the reaction speed and reaction selectivity, and reduces the amount of ortho-amino groups in the microchannel reaction process. The possibility of benzaldehyde or chloroacetone undergoing self-condensation, thereby improving the yield of 2-methyl-3-hydroxyquinoline.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
A kind of purification method of hexachloroacetone
ActiveCN109761776BLow impurity contentLow heating temperatureCarbonyl compound separation/purificationPhysical chemistryNitrogen gas
The invention relates to a method for purifying hexachloroacetone, belonging to the technical field of organic chemical purification. At 8.0KPa ~ 20KPa, after heating the crude hexachloroacetone solution to 45°C ~ 75°C, spray from top to bottom with the purification gas flowing from bottom to top heated to 35°C ~ 75°C for convective interactive contact purification, The low-boiling impurities are separated from the crude hexachloroacetone solution by purifying gas to obtain hexachloroacetone solution; the purifying gas is inert gas or nitrogen; and the low-boiling impurities are mainly pentachloroacetone and tetrachloroacetone. The method has a low material heating temperature, the purified gas can be recycled, the processing capacity can be enlarged, the operation is simple, and hexachloroacetone with extremely low impurity content can be obtained.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
A kind of preparation method of pymetrozine
ActiveCN108707137BShort reaction pathAtom economy is highOrganic chemistryOrganic synthesisMethyl palmoxirate
The invention discloses a method for preparing pymetrozine with an aim to provide a synthesis method short in reaction route of the pymetrozine, small in environmental pollution and simple in technology operation, and belongs to the technical field of organic synthesis. The method includes the steps of 1), allowing dimethyl carbonate serving as a raw material to be subjected to hydrazinolysis withhydrazine hydrate to obtain carbazide; 2), subjecting carbazide and smoke aldehyde to condensation reaction to obtain (E)-N'-(pyridine-3-kiya methyl) hydrazine carbon hydrazide; 3), subjecting (E)-N'-( pyridine-3-kiya methyl) hydrazine carbon hydrazide and chloroacetone to condensation reaction to obtain (E)-N'-(Z)-1-chloropropyl-2-subunit)-2-(pyridine-3-kiya methyl) diazanyl-1-carbohydrazide; 4), subjecting (E)-N'-(Z)-1-chloropropyl-2-subunit)-2-(pyridine-3-kiya methyl) diazanyl-1-carbohydrazide to ring formation under the alkaline condition to obtain the pymetrozine.
Owner:广东立威化工有限公司
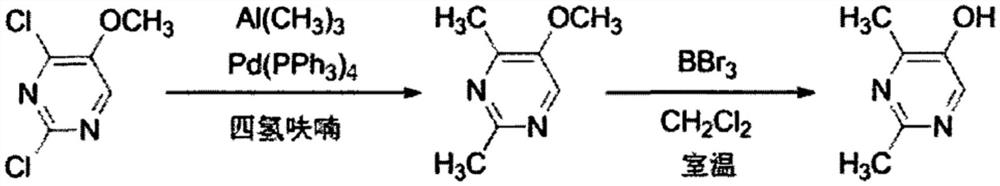
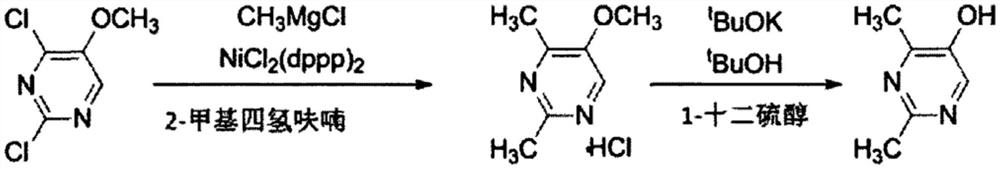
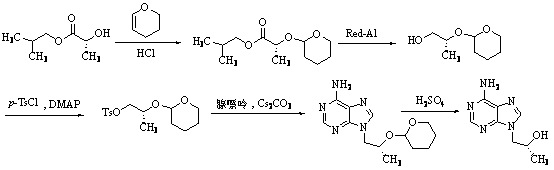
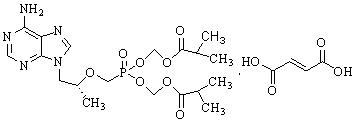
Method for synthesizing 2, 4-dimethyl pyrimidine-5-alcohol serving as intermediate of Leibolifera
The invention discloses a method for synthesizing 2, 4-dimethyl pyrimidine-5-alcohol serving as an intermediate of Lemborexant , and aims to solve the problems of safety risk and heavy environmental pollution of the 2, 4-dimethyl pyrimidine-5-alcohol in industrial production of the 2, 4-dimethyl pyrimidine-5-alcohol. The preparation method comprises the following steps: firstly, carrying out esterification reaction on benzoic acid serving as an initial raw material and chloroacetone; then, carrying out alkylation reaction and ring closing reaction with N, N-dimethylformamide dimethyl acetal and acetamidine hydrochloride; and finally, carrying out hydrolysis reaction to remove benzoic acid to obtain the 2, 4-dimethyl pyrimidine-5-alcohol. According to the method, benzoic acid widely existing in nature serves as a main raw material, the health problem caused in production is solved, the raw materials are non-toxic, and the method has the advantages that the EHS risk is low, the cost is low, the production efficiency is high, the yield is larger than or equal to 60%, and the purity is larger than or equal to 99.0%.
Owner:广西天铭药业有限公司
Method for preparing lorcaserin
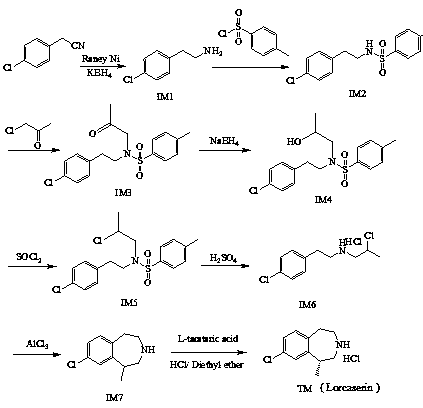
ActiveCN111499575AHigh purityRaw materials are cheap and easy to getOrganic chemistryChlorobenzeneEthyl group
The invention discloses a method for preparing lorcaserin. Specifically, the method comprises the steps: taking p-chlorophenylacetonitrile as an initial raw material, preparing p-chlorophenylethylamine through reduction; carrying out a reaction with p-toluenesulfonyl chloride to form an amino occupying intermediate; enabling the intermediate to carry out a reaction with monochloroacetone under analkaline condition to form N-(2-(4-chlorphenyl)ethyl)-4-methyl-N-(2-propionyl)benzenesulfonamide, and then carrying out reduction, chlorination, p-toluenesulfonyl removal and intramolecular Friedel-Crafts alkylation to synthesize 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzoazepine, carrying out L-(+)-tartaric acid resolution and alkalization on azepine to remove tartaric acid, and acting with hydrogen chloride diethyl ether to salify to prepare lorcaserin. The method has the characteristics of simple synthesis method, good reaction selectivity, high product purity, environmental protectionand low preparation cost.
Owner:HEBEI NORMAL UNIV
Simple synthesis method of 2-aminothiophene derivative
ActiveCN103739588BRaw materials are easy to getThe synthesis steps are simpleOrganic chemistryArylBiochemical engineering
The invention relates to a simple synthesis method of a 2-aminothiophene derivative. The method comprises the steps of condensating monochloroacetone, alkyl / aryl-piperzine under the effect of inorganic base to prepare a piperzine derivative II, mixing the piperzine derivative II with 4-chlorobenzoyl acetonitrile and sulphur, dropping organic amine, performing heat preservation reaction, and reacting in two steps to obtain 2-amino-3,(4- chlorine)-benzoyl-4-(N- alkyl / aryl-piperzine-N '-) methylthiophene. The simple synthesis method of the 2-aminothiophene derivative is available in raw materials, simple to operate, simple in synthesis steps and environmental-friendly; meanwhile, products are high in selectivity, yield and purity and applicable to industrial application.
Owner:XINFA PHARMA
1,1,1-trichloroacetone preparation method
PendingCN110857266AHigh purityControlling the position of chlorinationOrganic compound preparationCarbonyl compound preparationOrganic solventWater chlorination
The invention provides a 1,1,1-trichloroacetone preparation method, which comprises the following steps: 1, adding monochloroacetone and an alkaline compound into a large amount of an aqueous solutionwithout other organic solvents; 2, forming an aqueous solution or a turbid liquid from the alkaline compound in water while stirring; 3, introducing chlorine gas while stirring until the solution isclear; and 4, in the whole reaction process, controlling the temperature at 0-25 DEG C. According to the invention, the method is simple in process, convenient to operate, high in yield and convenientfor industrial production, and can effectively control the acetone chlorination position, so that the generation of byproducts such as 1,1,3-trichloroacetone, tetrachloroacetone, pentachloroacetone and the like is reduced, and the purity of the prepared product is high and can reach more than 85% without further purification.
Owner:ZHANGJIAGANG JIULI NEW MATERIAL TECH CO LTD
A kind of preparation method of hexachloroacetone
ActiveCN109942392BHigh yieldLow impurity contentOrganic compound preparationCarbonyl compound preparationPtru catalystNitrogen gas
The invention relates to a preparation method of hexachloroacetone, which belongs to the technical field of organic chemical gas preparation. Compound A and chlorine molecule B are mixed and reacted at 30°C to 150°C under the action of a catalyst, and the reaction product is a mixture containing hexachloroacetone and the catalyst; the catalyst is separated from the mixture to obtain a crude product; the crude product is purified to obtain Hexachloroacetone; compound A is at least one of acetone and chloroacetone with chlorine atoms of 1 to 5; chlorine molecule B is a mixture of chlorine or chlorine and a diluent gas, and the diluent gas is an inert gas or nitrogen; the catalyst is pyrimidine, 2-chloropyrimidine, 2-cyanopyrimidine or s-triazine. The method is easy to recover the catalyst, and can obtain the hexachloroacetone with extremely low impurity content in high yield.
Owner:派瑞科技有限公司
Purification method of hexachloroacetone
ActiveCN111517937AExtended service lifeEasy to operateCarbonyl compound separation/purificationAdsorption selectivityChloracetone
The invention relates to a purification method of hexachloroacetone, and belongs to the technical field of organic chemical purification. According to the method, pentachloroacetone impurities in crude hexachloroacetone are removed according to different adsorption selectivity of an adsorbent to pentachloroacetone and hexachloroacetone, so as to obtain hexachloroacetone with the pentachloroacetonecontent being 0.2wt% or below. The method is easy to operate, low in operation temperature, long in service life of the adsorbent and easy to amplify.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
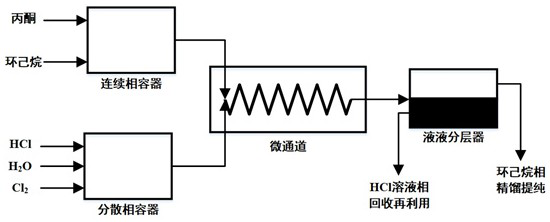
Method for continuously producing monochloroacetone by micro-droplet reactor
ActiveCN112358387AReduce generationEasy to purifyOrganic compound preparationChemical/physical/physico-chemical microreactorsAqueous solutionCyclohexanes
The invention discloses a method for continuously producing high-purity monochloroacetone by using a micro-droplet reactor. The method comprises the following steps: reacting acetone in a continuous phase with Cl2 in a dispersion phase by using a hydrochloric acid aqueous solution containing chlorine with a certain concentration as the dispersion phase and a cyclohexane solution containing acetoneas the continuous phase, and rectifying the obtained reaction product to obtain the high-purity monochloroacetone. According to the invention, an HCl solution containing Cl2 is sheared into micro-droplets by the continuous phase in the micro-channel, and the micro-droplets are surrounded by the continuous phase, so that corrosion of the HCl solution to equipment is avoided; and excessive acetonein the continuous phase reacts with Cl2 in the liquid drop on a phase interface to generate monochloroacetone, then monochloroacetone is diffused to a continuous phase main body, and Cl2 is insolublein cyclohexane, so that Cl2 can be effectively prevented from further reacting with monochloroacetone to obtain a by-product; and the high-purity monochloroacetone is continuously and efficiently produced by adopting the micro-droplet reactor, so that the generation of byproducts is greatly reduced, and the process is easy to control.
Owner:FUZHOU UNIV
Production method for hexachloroacetone
ActiveUS20160304428A1High yieldPromote recoveryOrganic compound preparationCarbonyl compound preparationActivated carbonCombinatorial chemistry
The present invention relates to a production method of hexachloroacetone, including performing a reaction between at least one kind of compound (A) selected from the group consisting of acetone and chloroacetones having a chlorine atom number of from 1 to 5, and a chlorine molecule (B) in a solvent in the presence of an activated carbon, to obtain the hexachloroacetone.
Owner:ASAHI GLASS CO LTD
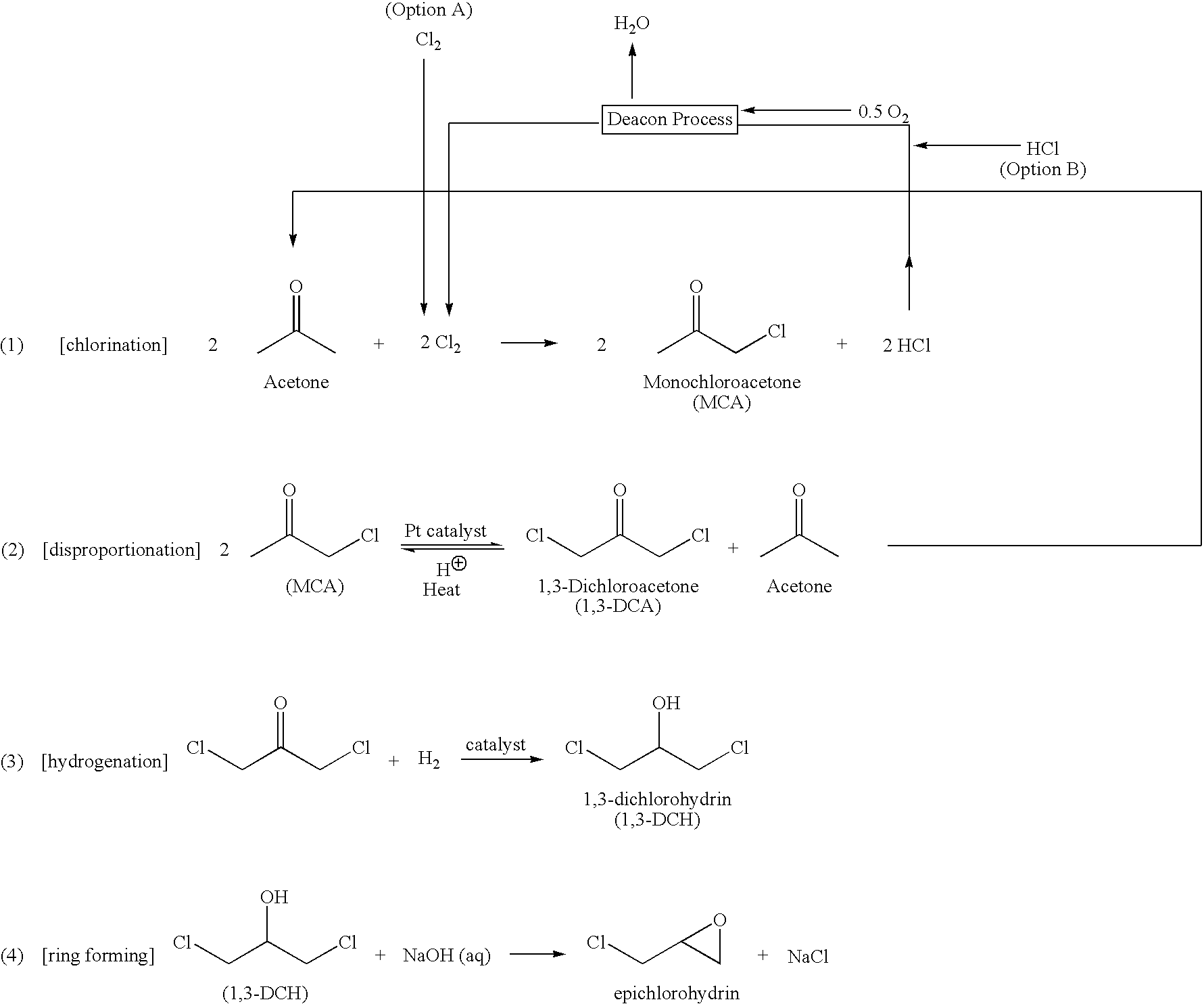
Process for preparing 1,3-dichloroacetone
Epichlorohydrin is produced from acetone by (1) chlorinating acetone to form monochloroacetone; (2) disproportionating the monochloroacetone in the presence of a platinum catalyst, a strong acid and preferably a chloride source (for example, added as a salt or from hydrolysis of monochloroacetone) and some water to produce acetone and 1,3-dichloroacetone; (3) hydrogenating the 1,3-dichloroacetone in the presence of a catalyst to produce 1,3-dichlorohydrin; and (4) cyclizing the 1,3-dichlorohydrin with a base to produce epichlorohydrin.
Owner:BLUE CUBE IP
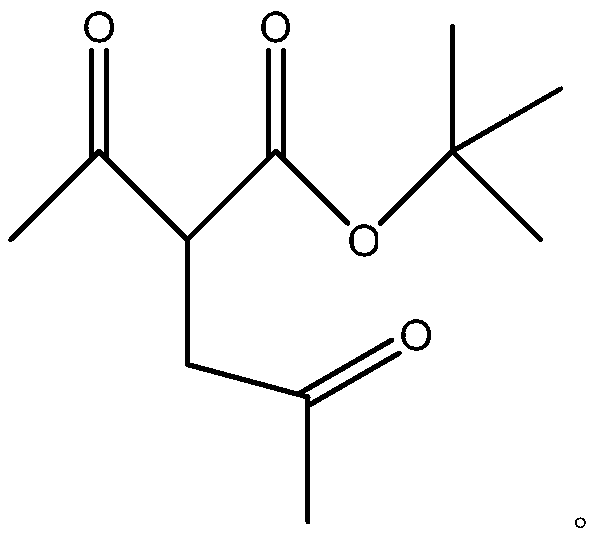
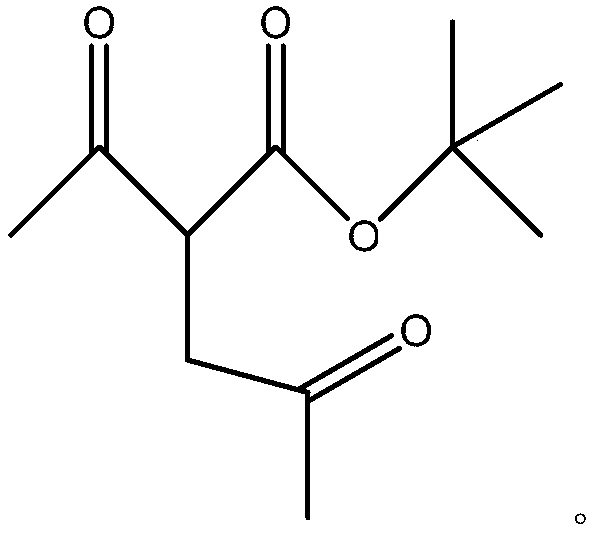
2,5-hexanedione synthesis method
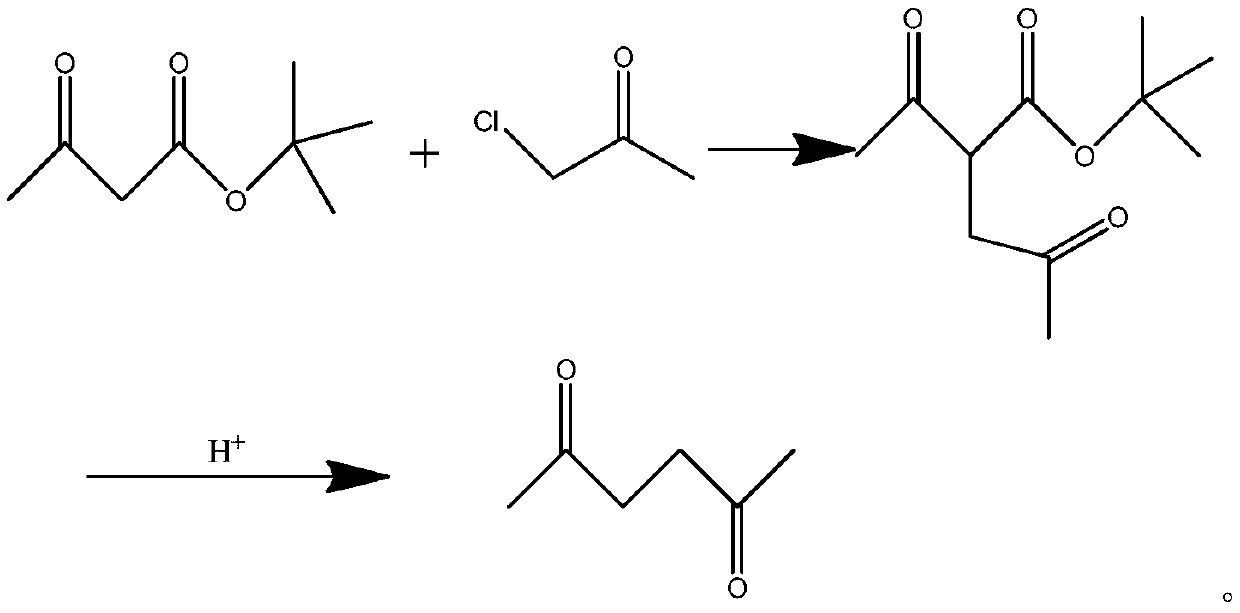
InactiveCN110845333AEasy to getLow technical costOrganic compound preparationCarboxylic acid esters preparationBiochemical engineeringTert-Butyl acetate
The invention provides a 2,5-hexanedione synthesis method, which comprises: carrying out a reaction on tert-butyl acetoacetate and chloroacetone under the action of an alkali to generate tert-butyl 3-acetylmethyl acetoacetate, hydrolyzing under acidic conditions, and deacidifying to obtain 2,5-hexanedione. According to the invention, the method has beneficial effects of low technical cost, easilyavailable raw materials and high yield.
Owner:HUAIHUA JINXIN NEW MATERIAL CO LTD
Method for preparing 4-methyl-2-sulfomethyl-1(3)H-imidazole
The invention relates to a method for preparing 4-methyl-2-sulfomethyl-1(3)H-imidazole. The technical problems of long route, low yield, difficulty in control of reaction, inconvenience in experiment operation and the like in the traditional synthetic process are solved. S-methylisothiourea (or various corresponding salts) and chloroacetone are heated to be prepared into the 4-methyl-2-sulfomethyl-1(3)H-imidazole, wherein K2CO3 serves as alkali and N,N-dimethylformamide serves as a solvent. The reaction formula is shown in the specifications. The 4-methyl-2-sulfomethyl-1(3)H-imidazole prepared by the method is a useful intermediate or product for the synthesis of a plurality of medicaments.
Owner:上海药明康德新药开发有限公司 +1
A method for purifying high-purity trichloroacetone for preparing folic acid
ActiveCN106946676BHigh extraction rateHigh purityCarbonyl compound separation/purificationSolventPharmacology
Owner:CHANGZHOU XINHONG PHARMA & CHEM IND TECHOLOGIES
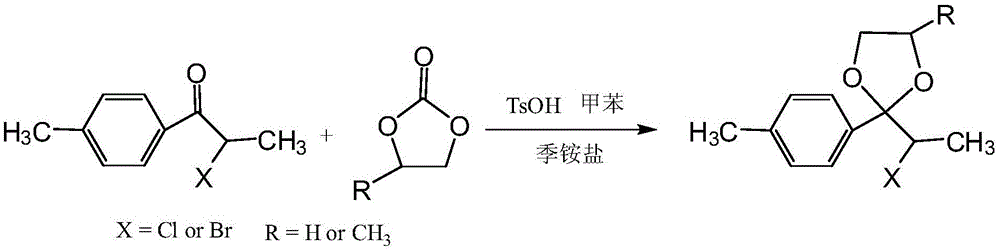
A novel process for synthesizing a ketal intermediate of 2-(4'-bromomethyl)phenylpropionic acid
InactiveCN105461678AEasy to synthesizeHigh selectivityOrganic chemistryPolymer sciencePropanoic acid
The invention belongs to the technical field of chemical engineering and particularly relates to a novel process for synthesizing a ketal intermediate of 2-(4'-bromomethyl)phenylpropionic acid. The process adopts a ketone and a cyclic carbonate as raw materials and prepares the ketal intermediate by performing ketalation under catalysis of a catalyst. The mole ratio of the ketone to the cyclic carbonate is 1:1-10. The mole ratio of the ketone to the catalyst is 1:0.001-0.2. The cyclic carbonate is ethylene carbonate or propylene carbonate. The ketone is prepared by subjecting methylbenzene to acylation with 2-halogen propionyl halogenide and is 1-p-methylphenyl-2-chloro-1-propiophenone or 1-p-methylphenyl-2-bromo-1-propiophenone. The process is characterized by high selectivity, a high conversion ratio and mild reaction conditions, can meet requirements of industrial production processes, and has a good application prospect.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
A kind of preparation method of cdk inhibitor
ActiveCN107118207BAvoid separation difficultiesEasy to scale up productionOrganic active ingredientsGroup 3/13 element organic compoundsS-methylthioureaPtru catalyst
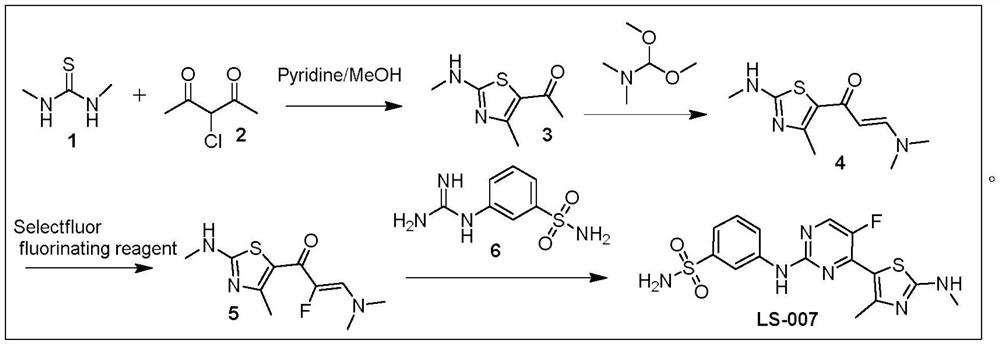
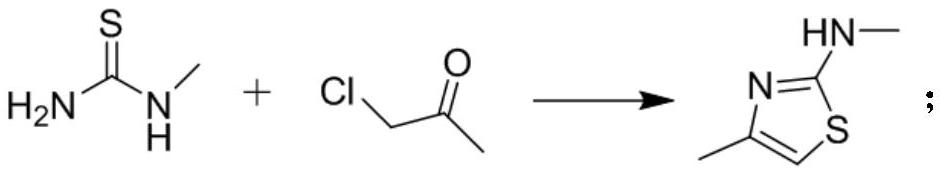
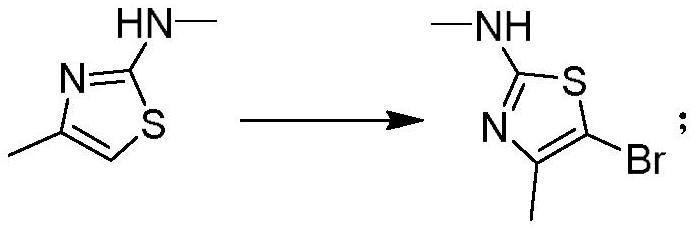
The invention discloses a preparation method of a CDK (Cyclin-dependent Kinase) inhibitor. The preparation method comprises the following steps: enabling monomethylthiourea and 1-chloroacetone to react to obtain N,4-dimethylthiazole-2-amine; carrying out bromination reaction to obtain 5-bromo-N,4-dimethylthiazole-2-amine; enabling the 5-bromo-N,4-dimethylthiazole-2-amine and borate to react under the action of a catalyst, so as to obtain an aromatic borate intermediate; enabling the aromatic borate intermediate and 2,4-dichloro-5fluorouracil to be subjected to suzuki coupling reaction under the catalysis of a palladium series catalyst, so as to obtain a coupled product; enabling the coupled product and aromatic amine to be subjected to Buchwald-Hartwig reaction to finally obtain a target product.
Owner:苏州东南药业股份有限公司
Preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid
ActiveCN109535083BIncrease productionImprove targeting selectivityOrganic chemistryMolecular sieve catalystsNitrosoCyanoacetic acid
The invention relates to a preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid. The preparation method comprises the following steps: enabling cyanoacetate and sodium nitrite to be subjected to reaction under the action of an organic solvent and / or an inorganic solvent and an acidic substance to obtain 2-oximidocyanoacetate; then, enabling the 2-oximidocyanoacetate andguanidine hydrochloride to be subjected to reaction under an alkaline condition to obtain 2,4-diamino-5-isonitroso-6-oxopyrimidine; enabling the 2,4-diamino-5-isonitroso-6-oxopyrimidine and hydrogen gas to be subjected to reaction under an alkaline condition by means of action of Pd / C to obtain 2,4,5-triamino-6-hydroxypyrimidine, and adding sulfuric acid to adjust the pH value to obtain 2,4,5-triamino-6-hydroxypyrimidine sulfate; and then, adding trichloroacetone and N-(4-aminobenzoyl)-L-glutamic acid to be subjected to reaction in a buffer solution under the action of a catalyst molecular sieve so as to obtain the folic acid. Based on the process route, the invention explores the influences of different reaction steps and refining conditions on the preparation route of 2,4,5-triamino-6-hydroxypyrimidine sulfate and the folic acid so as to reduce the wastewater pollution while increasing the yield.
Owner:ZHEJIANG BENLI TECH CO LTD
Method for preparing 1,1,3-trichloroacetone
ActiveCN108727173BHigh content of available chlorineChemically stableOrganic compound preparationCarbonyl compound separation/purificationPtru catalystOrganic synthesis
The invention relates to the field of organic synthesis and discloses a method for preparing 1,1,3-trichloroacetone. The method comprises: in the presence of an acidic catalyst, contacting a raw material containing 1,1-dichloroacetone and / or 1,3-dichloroacetone with symclosene for reaction. The method for preparing 1,1,3-trichloroacetone provided by the invention can achieve the purpose of obtaining 1,1,3-trichloroacetone with high purity and high yield in a short period in a pilot production process or even an industrial production process.
Owner:江西天新药业股份有限公司
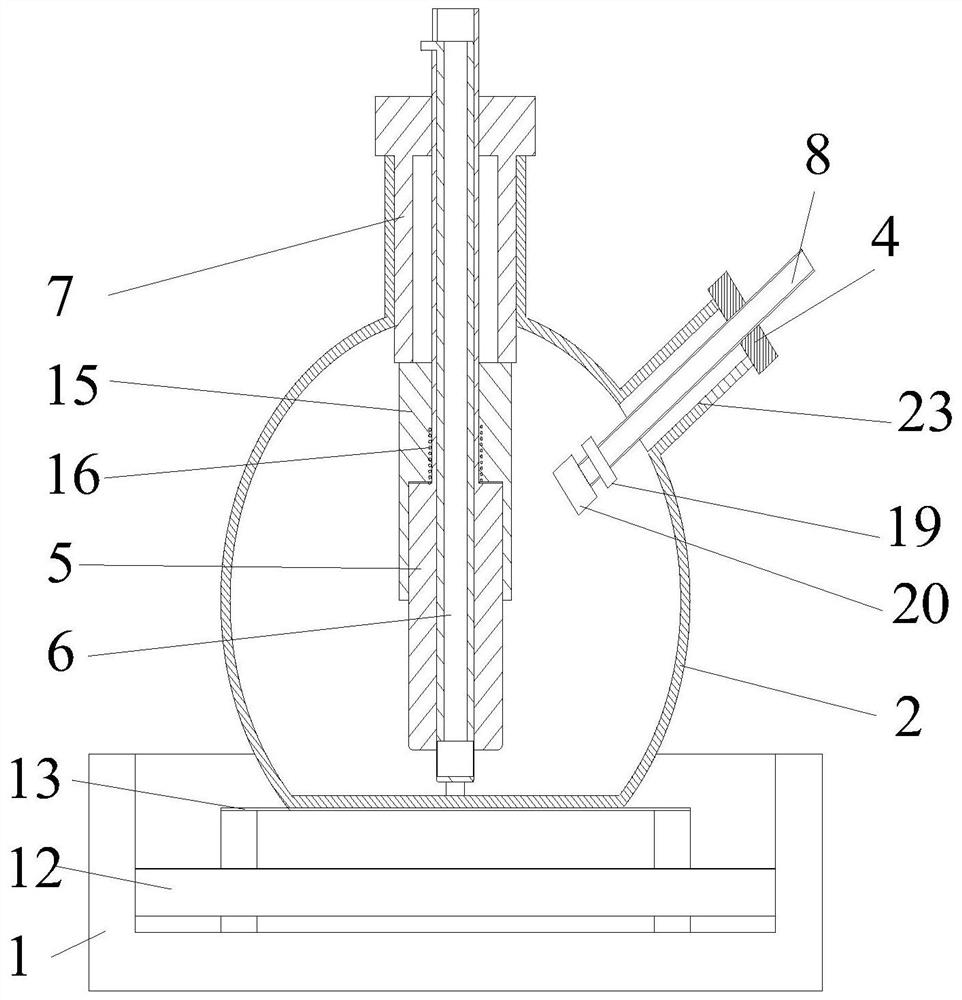
A kind of hydrodechlorination device and method for trichloroacetone
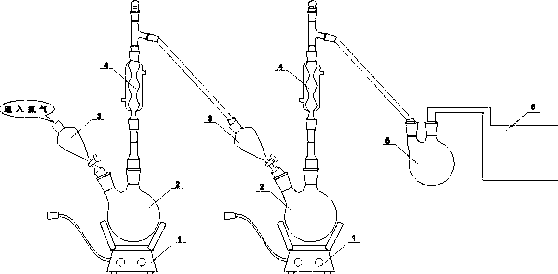
ActiveCN111644121BEliminate interferenceEasy to stretchCarbonyl compound separation/purificationChemical/physical processesEngineeringMagnetic stirrer
The invention discloses a hydrodechlorination device and method for trichloroacetone, relates to the technical field of chemical purification, and comprises a reaction vessel and a base, the reaction vessel is placed above the base, and the reaction vessel is provided with a first bottle opening, The second bottleneck, the third bottleneck and the fourth bottleneck, the second bottleneck is located at the center position, and a stirring structure is arranged in the second bottleneck; the stirring mechanism includes a stirring rod, a stirring rod arranged on the outside The end cover plug, the sliding sleeve, the stirring blade arranged at the bottom of the stirring rod and the connecting rod rotatably connected with the stirring blade, the feeding structure is arranged inside the stirring rod, and the upper end of the stirring rod is connected with the driving mechanism. This invention eliminates the The magnetic stirring bar interferes with the tubes in the reaction vessel when rotating, and improves the stirring efficiency at the same time, which is suitable for batch dechlorination treatment; and the stirring blades are easy to extend and retract, which is convenient for installation and disassembly.
Owner:CHANGZHOU XINHONG PHARMA & CHEM IND TECHOLOGIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/FDA0003018062530000011.png)
![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/BDA0003018062540000071.png)
![Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof Tricyclic furo[2, 3-d]pyrimidone compounds and application thereof](https://images-eureka.patsnap.com/patent_img/b8bdec14-229b-4335-aaf5-8d8349d7fe39/BDA0003018062540000281.png)